COVID-19 Related Regulations
22 June 2021
Disaster management adjusted regulations – 15 June 2021
With South Africa once again moving to lockdown level 3 as a measure to slow the third wave of COVID-19, SAMED has received queries relating to essential workers’ permits. These permits enable certain employees to work during curfew hours and undertake work-related travel during restricted hours. The permit is the same as that used previously and can be found at the end of the regulations.
13 July 2020
Updated Directions on the handling of COVID-19 Mortal Remains
Updated Directions on the handling of COVID-19 Mortal Remains
30 June 2020
Exclusion of certain alcohol-based hand-rubs from the operation of specific provisions of the Medicines & Related Substances Act
The Minister of Health, as supported by SAHPRA, has published provisions from which Category A medicines in class 13 or 20, consisting of alcohol-based hand rubs use or purporting to be suitable for the use to prevent or treat infection within a health establishment as defines in the National Health Act or other high-risk environments are excluded.
22 May 2020
Exclusion of medicines, medical devices and in-vitro diagnostics (IVDs) donated to the state from the operation of certain provisions of the Medicines and Related Substances Act
Dr Zwelini Mkhize, the Minister of Health, has published an exclusion on medicines, medical devices and in-vitro diagnostics donated to the State, or provided to the State as a sample as part of the submission of a bid for a tender published by the State, from provisions of section 18B of the Act: Provided that such donations are made or samples provided in accordance with the guidelines as determined by the Authority and relevant procedures required by the State.
This exemption shall be effective immediately for a period not exceeding three years from the date of signature of this Notice.
08 April 2020
Expansion of the scope of COVID-19 block exemption for the healthcare sector
The Competition Act exemption now also applies to medical and hygiene supplies (e.g. waste disposal products, sterilisation, prescription and non-prescription medicines, etc.), thereby allowing the actions (not price setting or price agreements) as authorised to each constituency under the initial exemption notice, e.g. to coordinate supply and stock levels.
24 March 2020
Covid-19 block exemption for the healthcare sector
These Regulations exempt certain agreements or practices solely aimed at responding to the COVID-19 pandemic. In other words, conduct that would ordinarily be caught by the cartel and vertical restrictive practices prohibitions of the Competition Act will now be exempt for the duration of the National State of Disaster. Notably, these Regulations come with the important caveat that any communication or agreement on prices between healthcare companies must be specifically authorised by the Minister.
The Regulations note that the management of the COVID-19 pandemic is not static and the Minister may expand the scope of these Regulations. The Regulations require that parties that take advantage of the Block Exemption keep minutes of meetings held and written records of any agreements struck or practices followed. Notably, even though the Regulations are effective immediately, any person may still make representations regarding the regulations within 14 days from their date of publication.
It is very important to note that where the application of an exemption, e.g. on procurement of supplies by hospitals, take place, no price-setting would be permitted, and that price negotiation to lower costs must take place under auspices of the Minister of Health.
We, therefore, urge members to be extremely cautious when implementing these exceptions and to seek assistance before commencing, with “communication”, “coordination”, “transfer” or “procurement” in a collective fashion. The exemptions can be summarised as follows :
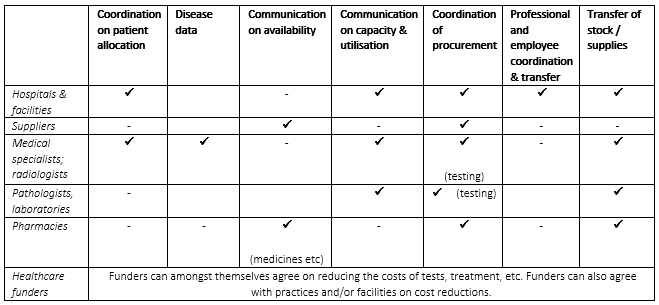
24 March 2020
Consumer and customer protection and national disaster management regulations and directions
These Regulations aim to protect consumers from unlawful pricing behaviour in respect of products and services that are critical to effectively responding to the COVID-19 pandemic. These products and services are listed in the annexures. These Regulations put in place two measures to avoid unlawful price increases in respect of goods listed. First, the Regulations introduce the following new considerations that will count towards an assessment of whether a dominant firm has engaged in excessive pricing. Second, the Regulations go on to state that price increases that have either of the abovementioned characteristics will be deemed to be unconscionable, unfair, unreasonable and unjust in terms of the Consumer Protection Act (the “CPA”).
Notably, these Regulations provide that the Minister may, in consultation with the Minister of Health, issue directions to set maximum prices on medical goods and services relating to the testing, prevention and treatment of COVID-19 and its associated diseases during the National State of Disaster. Dominant firms which contravene these Regulations must be investigated by the Competition Commission and, if found to be in contravention, will be liable in accordance with the penalties provided for in the Competition Act. The Regulations go on to set in place the general penalties for a contravention of these Regulations, subject to the Competition Act and the CPA.
