Medical Technology Code

Professional integrity is at the heart of quality healthcare, and the global
healthcare industry is carefully scrutinised and highly regulated.
There is close collaboration between the medical technology industry and healthcare professionals in utilising technological innovation to improve patient care. Healthcare professionals play a vital part in research and development leading to medtech innovation, and suppliers provide training and service support to health professionals to enable them to use products correctly.
In the context of this mutually beneficial relationship, it is critical to keep sight of the fact that the patients’ interests must always come first. In particular, medtech companies must: (i) respect the obligation of healthcare professionals to make independent decisions regarding patient treatment; and (ii) guard against inappropriate and irresponsible marketing and business practices which could compromise patient outcomes and damage the reputation of healthcare professionals and the industry.

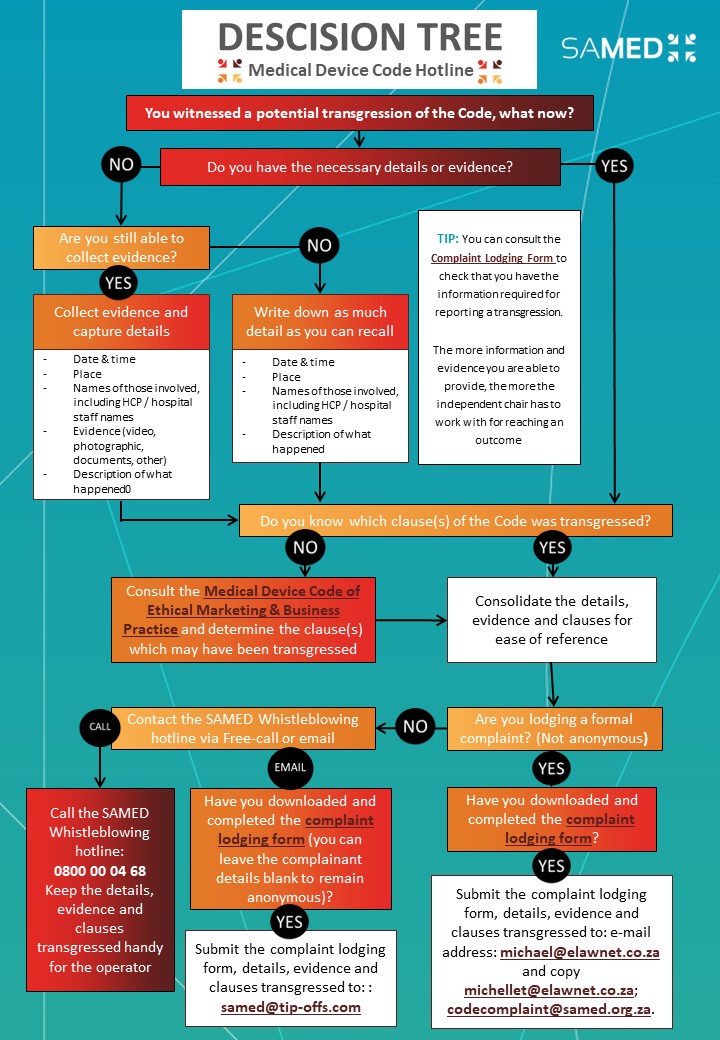
The Medical Technology Code for Ethical Marketing and Business Practice (the Code) governs interactions between suppliers and their customers, namely healthcare providers and professionals. It fosters trust in the medtech industry by creating a framework for ethical activity that protects the reputation of industry stakeholders.
The Code is an instrument for self-regulation of the medical technology industry and SAMED is the custodian of the Code. Its key principle is that signatories to the Code will not offer inappropriate inducements to healthcare providers and healthcare professionals in order to sell or lease their products.
Penalties may be imposed on signatories to the Code if, after a fair investigation and/or hearing, they are found to have transgressed the provisions. This process is triggered by a complaint or tip-off to the Medical Technology Code Hotline (free call 0800 00 04 68; email: samed@tip-offs.com).
List of related reporting mechanisms:
- Special Investigating Unit (SIU): Report health sector corruption on 0800 037 774
- Board of Healthcare Funders (BHF): Report instances of fraud, waste and abuse on medical insurance schemes on 080 847 4368.
- Council for Medical Schemes (CMS): Report suspected fraud against an employee or member of Council for Medical Schemes on 0800 867 423 or cms@behonest.co.za
- Health Professions Council of South Africa (HPCSA): Report suspected fraudulent acts, corruption or irregularities by any of member, employee or supplier of the HPCSA on 0801 122 565 or hpcsa@tip-offs.com
- Section 27: Report rights violations on 066 076 8845
- Corruption Watch: Report abuse of public funds, assets, or position. Corruption to get a tender and tender irregularities. Bribes. on 0800 023 456 or via the link
- Organisation Undoing Tax Abuse (OUTA): Report: Squandering, maladministration, and misuse of taxes via the link
- Competition Commission: Report: Breach of competition law by completing the form
- Office of Health Standard Compliance (OHSC): Report Complaints about poor healthcare from patients and their family members and from employees in the healthcare system on 080 911 6472 or complaints@ohsc.org.za
- South African Health Products Regulatory Authority (SAHPRA): Report unlicensed health product suppliers/companies and medicines on mokgadi.fafudi@sahpra.org.za
- South African Medical Association (SAMA): Report poor service and standards at public and private health facilities on whistleblower@samedical.org
- Financial Intelligence Centre: Report money laundering and the financing of terrorism on 012 641 6000 (option 1) or via the link
- Compensation Fund: Report compensation fund fraud on 0800 234 432 or sms 30916 or cf-fraud@thehotline.co.za
- National Health Laboratory Services (NHLS): Report fraud and corruption on 0800 000 552 or sms 30916 or nhls@thehotline.co.za
- South African Revenue Services (SARS): Report tax crime or non-compliance on 0800 00 7277
- National Department of Health (NDoH): Report fraudulent/fake tender and supply requests on 012 395 9591 or majap@health.gov.za