SAMED Policy Paper
COVID-19 Vaccine roll-out
April 2021
Introduction
As the spread of the coronavirus COVID-19 continues to impact the health of people and the economy of South Africa, the South African Medical Technology Industry Association (SAMED) and its members are working to support efforts against the pandemic including the rollout of the COVID-19 vaccine(s). We are committed to collaborating with all stakeholders in support of the need to contain and combat the COVID-19 pandemic.
This policy paper draws on best practices and global learnings and is likely to evolve with the progression of the pandemic.
Contents:
- Ancillary medical technology required for the vaccine rollout
- Factors to consider for vaccine delivery
- Global demand and local supply/manufacture of COVID-19 vaccine-related medical technologies
- Quality, standards, testing and vetting of suppliers
- Supply chain integrity
- Distribution considerations
- Medical device representatives needed in critical areas included in phase 1
- General considerations
- Reference documents to consider
1. Ancillary medical technology required for the vaccine rollout
The following is a list of possible ancillary medical technology that may be required:
- Personal Protective Equipment (PPE) ie gowns, overshoes, gloves, masks and face shields worn by vaccinators
- Syringes
- Needles
- Safety boxes/sharps containers
- Vaccine carriers
- Cooling packs
- Cold Storage Solutions e.g. fridges, ultra low-temperature freezers
- Temperature monitoring systems
- Markers
- Hand sanitizer
- Swabs
- Cotton wool
- Ancillary kits: Some vaccines are accompanied by ancillary kits (including some or all of the above items), others not.
2. Factors to consider for vaccine delivery
a) General
- A COVID-19 vaccine, like many new vaccines will most likely be in a multidose vial probably enough for 10 patients initially. Launching new vaccines in multidose vials make it available faster and for more patients.
- Prefilled syringes may be a subsequent stage.
- The vaccine(s) could either be a “prime and boost’’ concept, two shots with a few weeks in between or one shot.
- Injectable drugs and majority of vaccines cannot be administered without needles and syringes.
- Vaccines like most injectable drugs are administered by 1 ml syringes and commonly done via intramuscular injection. The COVID-19 vaccines doses are less than 0.5 ml and therefore most likely to be delivered via the intradermal route.
- We know that adequate quantities to address a full-scale vaccination campaign are not likely to be readily available.
- It is, therefore, crucial to have a plan indicating timelines and quantities of delivery devices required.
- Suppliers should have the capability to provide healthcare workers with easy access to virtual training tools enabling them to improve safety and efficiency before vaccination campaigns start.
- For every dose administered it is anticipated that delivery devices materials will be required for the following stages:
o Preparation (swabs, needle + syringe + optional vial access device with multi-dose vials)
o Administration (needle + syringe or combination)
o Sharps collector. - Additional needles and syringes will also be required for preparation, but these volumes would depend on the type of vial that the vaccine comes in.
- Given the infectious nature of COVID-19 and the large volume of people that will be vaccinated at any one time, as well as the high prevalence of Human Immunodeficiency Virus (HIV) among the South African population, it is recommended that devices with safety technology be used.
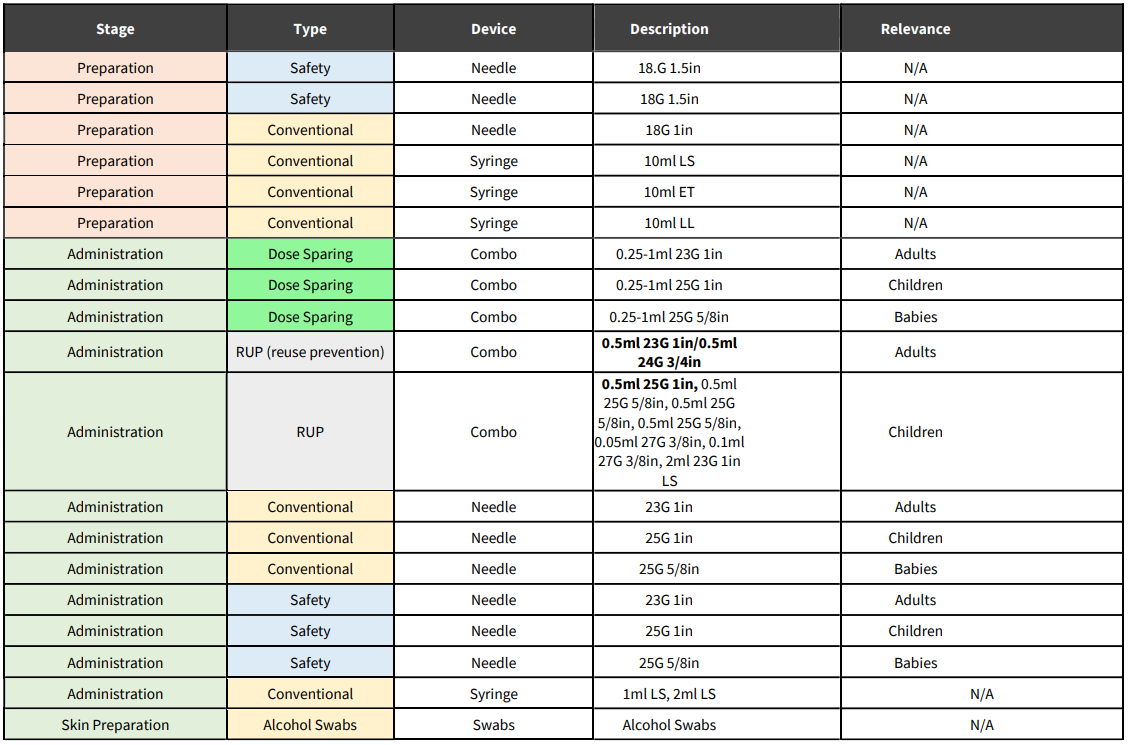
- Dose sparing technology should be considered.
- Given the potential local manufacture of vaccines, pre-filled syringes also need to be considered for future delivery.
- Some of the available technologies for each stage are outlined in the table below.
b) Ensure safe vaccination delivery
Providing additional and refresher training for vaccinators on the importance of safe injection practices will be especially important to ensure vaccination safety. Additional injections will also increase the quantities of safe injection supplies needed. Budgeting for these additional supplies, including infection
protection control measures, to ensure their timely availability is an important step in the planning process. Other vaccination sites and healthcare workers could be used to assist with the vaccinations, for example pharmacies and corporate clinics with existing trained staff and protocols for cleaning and
sanitising will assist the vaccination procedure.
c) Safeguard injection safety
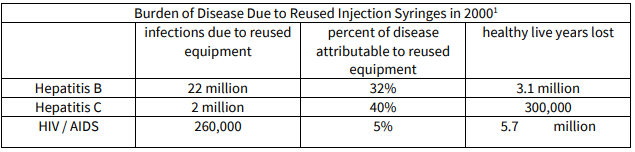
Injection safety is the safe handling of all injection equipment, routine monitoring of the availability and use of safe injection equipment, and correct disposal of contaminated injection equipment. Sharps and, more specifically, needles are considered the most hazardous category of healthcare waste for health workers and the community at large if they are not properly handled and disposed of. Waste management of biohazardous materials i.e. following universal precautions for handling of sharps as well as disposal of biohazardous materials at vaccine administration clinics/sites is critical. Certified/approved waste removal companies must be engaged for this destruction process. Needlestick injuries can easily occur and carry a high potential for infection, including hepatitis B and hepatitis C, HIV and sepsis. To prevent risk of infection to the community and to health workers, the safe disposal of used needles and syringes is a critical component of any immunization programme. To prevent risk of infection to patients, community and to health workers, safe injection practices are vital. Proper injection technique, the use of safety devices and the safe disposal of used needles and syringes are fundamental components of any immunization programme. An adequate supply of safety boxes and their proper disposal must be assured.
The World Health Organization (WHO) provides an online training course on standard precautions for injection safety that could serve as a timely refresher for those administering injections in the context of COVID-19. In addition to the traditional injection safety recommendations, in the context of COVID-19, vaccinators should perform hand hygiene after each recipient, with soap and water or hand sanitizer containing 60–80% alcohol to help prevent the spread of COVID-19.
d) Cold chain equipment considerations
It is recommended that the National Department of Health (NDoH) consider an Inventory and Gap Analysis Tool for assessing vaccine volumes and corresponding cold chain capacity per catchment area. Identifying surge capacity: (i) assess and map available cold chain capacities according to the three temperature ranges (eg +2 °C to +8 °C, -20 °C, and -70 °C) for storing the different types of COVID-19 vaccines (ii) all available cold chain equipment outside the immunization programme (eg pharmaceutical division, national reference laboratories and private and business sectors).
3. Global demand and local supply/manufacture of COVID-19 vaccine-related medical technologies
To assist with forecasting and tendering, an analysis needs to be done as to who is currently supplying these technologies, where they are being imported from and at what capacity, including potential global supply constraints or challenges.
It is recommended that a data collection exercise be implemented to determine current and future local capacity and if relevant, potential for local manufacturing. There may not be enough capacity in the global industry to manufacture the required amount of syringes and needles in a short period of time.
Planning ahead will provide the best opportunity for the global industry to manufacture the number of devices required to be ready for mass vaccination programmes. It is also crucial that the current immunization schedules for other diseases such as Bacillius Calmette-Guërin (BCG) not interrupted due to a shortage of delivery devices. For this reason, it is of utmost importance that the National Department of Health (NDoH) determines its requirements for delivery devices as soon as possible and places orders with the delivery device importers and local manufacturers in a timeous manner.
Medical technologies currently being locally manufactured include: masks, face shields and hand sanitizer. Among other items worthy of consideration for local manufacturing are syringes, needles and sharps boxes. Factors that support/deter local manufacturing need to be identified so that related policies are amended and targeted incentives designed and implemented in order to allow for sustainable ongoing local manufacture. Such factors include resolution of regulatory hurdles, resources for implementing quality management systems, lack of testing and sterilization services, tariff reviews and policies that drive commitment by National Treasury and national and provincial government departments to buy locally manufactured products even if these entail a higher price than that of imports. It is worth noting that important lessons can be obtained from other countries that increased local manufacturing capacity of medtech and which dealt with similar challenges.
4. Quality, standards, testing and vetting of suppliers
a) Only South African Health Products Authority (SAHPRA) licenced suppliers should be considered in the procurement process.
b) A rigorous vetting process should be implemented by the National Department of Health (NDoH) in collaboration with South African Health Products Authority (SAHPRA) and industry experts.
c) Testing methods currently implemented by the National Department of Health (NDoH) must be adhered to and maintained for all suppliers. In the absence of local testing capacity, it is recommended that the National Department of Health (NDoH) consider identifying internationally accredited testing laboratories and acceptance of their testing results.
d) Internationally recognised standards and specifications should be identified for each product type and only those products that meet such standards should be procured. Food and Drug Administration (FDA), Conformitè Europëenne (CE)marking, International Organization for Standardization (ISOaccreditation) or World Health Organization (WHO) approved devices will provide assurance that quality standards are being met. For example, in the case of needles and needles, the relevant standards would be SANS 1124-1 and SANS 1124-2 (South African National Standard).
5. Supply chain integrity
a) Reinforcing supply and stock management: Monitoring and recording of cold chain equipment temperature, vaccines distribution, inventory and stock management, wastage rates should be done rigorously and efficiently throughout the supply chain.
b) Procurement: Consider the value-based procurement (‘VBP’) concept which focuses on purchasing medical technology based on its longer-term overall value rather than on its upfront cost, thus forming a powerful means for improving patient outcomes and/or reducing the total cost of care delivery. VBP focuses on the value that a particular product/service/solution can create, in terms of improved outcomes for patients, cost of care efficiencies and benefits for other stakeholders, leading to economic most advantageous purchasing. See: https://www.euriphi.eu/innovationprocurement/value-based-procurement/.
c) Measures to curb and combat corruption and overinflating of prices must be considered. One safeguard is that suppliers be signatories to the Medical Device Code of Ethical Marketing and Business Practice. Consideration should be given to using the open contracting data standard (see https://standard.open-contracting.org/latest/en/). This will minimize opportunity for corruption. It is recommended that procurers consider the length of time that a company has been manufacturing or supplying the type of product required. New entrants must be vigorously vetted, and the same stringent regulatory processes applied.
d) Centralised procurement may have shortcomings that need to be considered i.e. an efficient and costeffective central procurement process requires a single national data base of all medical items, which is continuously updated regarding stock levels at every healthcare facility. Excessive use of centralised procurement risks impeding competition (monopsony power) and can reduce participation of small- and medium-sized enterprises (SMEs) as they may not have a national footprint.
e) Procurement mechanisms should align with / take into account current processes, skills and infrastructure or lack thereof.
f) Training: Suppliers must provide ongoing training as part of the contract. This is especially important when it comes to vaccination administration and reuse prevention. See the following table:
g) Competitive tendering and encouraging supplier diversity:
- Consideration should be given to SMEs, acknowledging that some of these suppliers might not have a national capability.
- Suppliers must be timeously reimbursed.
- To meet different service provider and patient needs, medical technology procurement contracts should not be exclusive, but should allow for participation of multiple supplier models and types where feasible.
- Tendering processes should not be implemented in a way that artificially controls the number of competing organisations. This principle should be reflected in limits to the size and duration of tender contracts, so as not to create or perpetuate market monopolies.
- Where competitive tendering is used, a maximum threshold should be established to limit the proportion of purchasing that could be bundled under a tendering process.
- As most medical technology markets are highly competitive, national sole source contracting practices are not appropriate for most product categories. Multiple source contracts are generally preferable so that a diverse range of products and services is available. Multiple-supplier contracts allow a larger number of suppliers into the market, which strengthens competition and ensures stability of supply. Less competition also reduces the procurer’s negotiation leverage in future rounds of purchasing, ultimately resulting in higher long-term procurement costs.
- International tendering is not advised as this may result in quality issues, distribution issues, lack of back-up support and training on the ground – activities that existing local suppliers are well versed in.
6. Distribution considerations
a) A distribution plan should be prepared for vaccines and ancillary supplies, based on the target population and number of staff that will comprise the vaccination and monitoring teams (e.g. vaccinators, recorders, social mobilizers, supervisors and monitors). Distribution across the country will require well considered logistical coordination – some vaccines may have stringent refrigeration requirements, generally only found in research laboratories. Others may require less onerous refrigeration, but still a cold chain – in a country where reliable electricity is a challenge. Getting this right will require skilled planning.
b) Vaccines will need to be delivered to and properly stored at clinics, pharmacies and all health facilities, public and private. South Africa has a wide and well-functioning network of such facilities. Logistical coordination and support from all sectors of society will have to be mobilised. Suitably qualified organisations and/or Non-Governmental Organizations (NGO’s), for example, the Red Cross, Gift of the Givers, Doctors without Borders (MSF), South African National Blood Service (SANBS) and others with technical skill and capability must be enlisted in this effort, as well.
c) Additional volumes of relevant ancillary medical technology for training purposes must be considered, as well as those that might need to be discarded of due to adverse events.
d) If a two-needle technique is used (for a multi-dose vial) then additional needles are required for preparation. If a single needle technique is used, then a vial access device will be needed for a multidose vial. Risk associated with a single needle technique is that the tip of the needle could become blunt if used twice (i.e. for extracting the dose from the vial and vaccinating the patient) which can cause pain to the patient.
e) Note: Procuring the sheer number of needles and syringes needed to support an operation of this size involves complex logistics, which is why vaccine administrators may see variance in how the supplies are packaged. The products may be received as a combo, defined as a single packaged needle and syringe, or as individual components. Needles and syringes for vaccine administration may be packaged as integrated units (i.e. NO assembly required) or as separate items in a kit (i.e. assembly required).
f) Cold chain and storage conditions must be maintained throughout the distribution cycle.
g) Security of vaccinations must be ensured throughout the distribution cycle and will require collaboration across the value chain and with other government agencies/ministries.
7. Medical device and IVD representatives needed in critical areas included in phase 1 of the Covid vaccination rollout
Healthcare workers, the elderly, other front-line essential workers (including medical device representatives needed in theatre/surgery and healthcare facilities to support treatment of patients) and those with comorbidities should be the first recipients of the vaccine. Ideally, the most vulnerable to the least should be vaccinated in a systematic rollout over the subsequent months following the receipt of vaccine stock. Healthcare workers and other essential workers should get their vaccine through workplaces. For the elderly, strategies to vaccinate them will include deploying nurses and other qualified medical staff to offer vaccination at pension points, in communities and primary healthcare centres where people with chronic illnesses like diabetes and hypertension get their medication. Mass immunisation strategies in rural areas would have to be developed at district level in recurrent rollout programmes. Using existing networks, such as private pharmacies, makes sense for broader distribution.
SAMED recommends that medical technology industry company representatives that perform critical functions be included in the initial phases based on their direct risk of exposure and to ensure continuity of patient care throughout the healthcare system. These personnel, often referred to as ‘Company Representatives in the Clinical Environment’ (CRICE) are required, among others, to:
- Be present in patient care settings to provide technical support concerning the safe and effective application of surgical products and technologies.
- Support procedures/equipment/technology in the operating room or procedural suite and are required to be present during urgent, non-elective procedures (e.g. trauma, transplant, cardiac treatment) and other medically necessary procedures (e.g. joint replacement, cancer treatment, dialysis etc).
- Be involved in the remote calibration or adjustment of medical devices (for example, pacemakers, laser technology) to the surgeons’ and manufacturers’ specifications.
- Service or repair critical medical devices and equipment (including diagnostics).
In these settings, they are at direct risk of exposure to patient specimens, including blood, tissue and other biological materials. Personnel essential to manufacturing activities of vaccine delivery ancillary medical technologies and all other medical technologies should also be considered for the vaccine in subsequent early phases.
8. General considerations
The benefits of being vaccinated must be visible and noticeable to encourage people to get vaccinated – some vaccine candidates need to be given twice, a few weeks apart. To get to the levels of immunity we need to achieve, known as population immunity, where there is sufficient suppression of the virus to allow near-normal activity to resume, scientists advise that 60-70% of the population needs to develop immunity. For young children, leveraging the South African vaccination programme is recommended. We must follow global best practice and innovate locally to reach scale. To be able to vaccinate 60-70% of the population will require deep collaboration among social partners and expert coordination.
9. Reference documents to consider
World Health Organization (WHO) Guidance on developing a national deployment and vaccination plan for COVID-19 vaccines. See: https://www.who.int/publications/i/item/WHO-2019-nCoVVaccine-deployment-2021.1-eng
1 WHO Global Burden of Disease Study, 2000, as cited in “A Procurement Guide: Procuring Single-use Injection Equipment and Safety Boxes”, April 24, 2003, WHO
