SAMED Position Paper
Procurement of Medical Technology
September 2021
Introduction
SAMED recognises that effective procurement of medical technology makes an enormous contribution to the quality of care offered by the health system to the public. This paper sets out SAMED’s position on the procurement system required to optimise the contribution of medical technology.
In general terms, SAMED argues for a procurement system that is ethical, efficient, and effective and that promotes competition in the market. More specifically, SAMED promotes the goal of a procurement system that takes account of the unique characteristics of medical technology. At the heart of such a system is the concept of value-based procurement.
Background
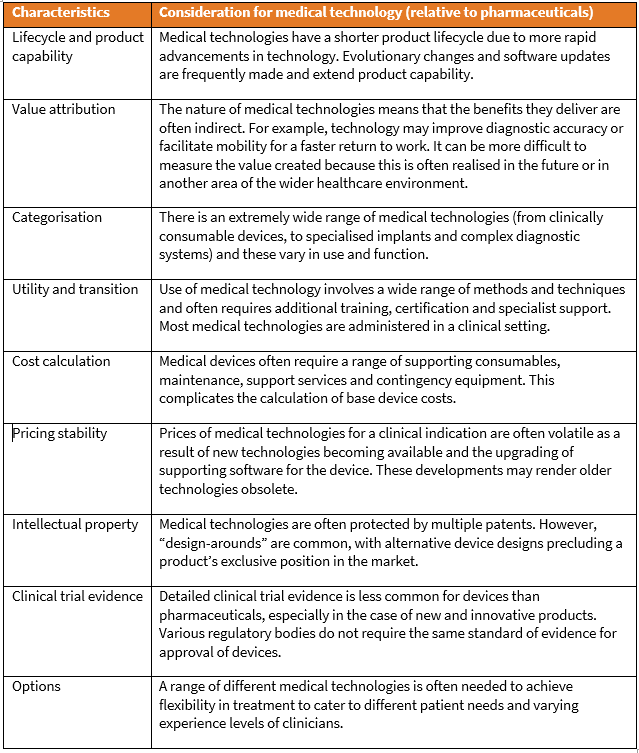
Medical technology poses unique challenges to procurement systems and these need to be understood. Major considerations are outlined briefly below.
- Medical technology undergoes rapid cycles of improvement and requires variation to meet individual patient and healthcare professional needs. Devices are often not standard commodities. A medical technology procurement system needs to take account of the product improvement cycle as well as the need to accommodate clinical variation among patients. A system that considers only the lowest price will tend to favour older technology and may eliminate models which would meet different clinical and patient needs. Procurement planning should allow for:
- Improvements to be introduced within contract structures in order for patients to benefit from innovation.
- Non-exclusive contracts, allowing for multiple models and types, to meet the needs of different healthcare providers (HCPs) and patients.
- Medical technologies often remain implanted in a patient or in use at a hospital for many years. Much of the cost and economic value of medical technologies lies not in the purchase price but in servicing, technical support, training and education provided by the suppliers. For example, much of the service and support for implantable devices is done after implantation. Additional hospital procedures to remove, replace or adjust devices add greatly to overall costs. To be cost efficient, a tendering system must take into account the value of the device over the duration of the patient’s clinical condition.
- Maintenance of medical equipment is critical. Procurement managers should be wary of suppliers who offer a good price, but do not provide maintenance services or guarantee availability of spare parts. Some medical devices require additional equipment to function, and a supplier should be willing to provide and support such equipment. The goal for any medical equipment is to ensure full functionality across its life cycle.
- The consignment model may sometimes be more appropriate than direct purchasing. In some surgery it is impossible to establish upfront the exact type and size of device or implant required, and a range of products must be available to the surgeon as options. A clear and efficient procurement-to-payment system needs to be established to address the unique process of consignment inventory. The main control in such system is validation of the products actually used during the operation.
- Appropriate and efficient purchasing and procurement will be critical to the success and sustainability of the reformed health system funded mainly by National Health Insurance (NHI). There is a perception that central procurement of medical technology is a panacea to drive down the costs of technology in healthcare. This model of centralised procurement is often applied to pharmaceuticals. However, medical technologies have characteristics that differentiate them from pharmaceuticals, and the model used for procuring pharmaceuticals cannot be simply copied for medical technologies.
The table below lists specific considerations that apply to medical technology tendering.
The case for value-based procurement
Value-based healthcare is a concept intended to contribute to more efficient and sustainable health systems. The approach considers the life-cycle cost of healthcare delivery and wider outcomes of care for the patient and society. It can result in more economically advantageous solutions while increasing the quality and value of care for patients, HCPs and health systems.
The United Nations’ procurement division defines best value for money as “the optimisation of whole life costs and quality needed to meet user’s requirements, while taking into consideration potential risk factors and resources available”.[1]
In the European Union, value-based purchasing is facilitated in accordance with the “most economically advantageous tender” (MEAT) approach. This approach adopts a broad definition of value, taking into account the benefits of a particular product/service/solution in terms of improved outcomes for patients, cost of care efficiencies and benefits for other stakeholders – the sum of which is the most economically advantageous purchase.
These terms and practices are gaining ground in various jurisdictions as health system managers have become aware of the negative results of procuring solely on lowest price.
Procurement practices are a critical factor in implementing value-based healthcare. The treatment options identified by procurement managers impact directly on the care available to patients. Unfortunately, current healthcare procurement tends to focus too strongly on the purchase price of products and underplay considerations of quality, safety, effectiveness and – very importantly – cost-effectiveness of the technology.
What is value?
The World Health Organization (WHO) has defined value in healthcare as follows: “A healthcare system that delivers value for money is defined as one that maximises efficiency, enabling the population to attain the highest possible level of health given the level of expenditure.”[2] Value, therefore, encompasses both cost and non-cost factors.
Cost-related factors extend beyond the initial purchase price and accrue across the life cycle of many medical devices. They include:
- Direct medical costs, such as diagnostic tests, fees of HCPs, hospitalisation and sub-acute care.
- Costs of maintaining, cleaning and storing the device.
- Ongoing operating cost.
- Upgrade costs.
- Staff training and other personnel-related costs.
- Decommissioning and disposal costs.
The non-cost value of a medical device incorporates factors associated with patient outcomes which often result in total budget savings, including:
- Delivery efficiencies.
- Technical benefits and merits.
- Safety in terms of reducing adverse events or complications.
- Clinical effectiveness, including reductions in morbidity or mortality, and patient preference and satisfaction.
- Reliability and quality of support by the vendor or manufacturer, including warranty, maintenance, customer care, and clinical training and support.
- Societal benefits, such as improved quality of life for the patient, reduced loss of productivity, and lower social care requirements.
- Environmental effects, for example, in terms of the sustainability of the technology.
Value-based purchasing is largely about broader patient health and the societal benefits conferred by a medical device. To achieve value, procuring authorities should focus on spending well, rather than spending less.
Ensuring that all aspects and levels of value are considered, and passed on to the patient, can increase the financial stability of the health system and its responsiveness to patient needs.
Assessment of the value of a medical device should consider the following crucial information inputs[3]:
- The perspective from which the technology is being evaluated – for example, the perspective of a healthcare funder, HCP or health authority.
- The pace of innovation for the device category. Many device categories undergo frequent incremental improvements.
- The potential for the device to reduce expenditure on other healthcare products and/or services. Some innovative devices may reduce length of hospitalisation or simplify/eliminate related procedures and their associated expense.
- The opinion of HCP who would use the devices. It is important that the views of HCPs on the value of a device in the prevailing medical context be included in the assessment.
Innovation as a factor in value
Medical technology innovation is about finding new approaches in order to improve patient outcomes, enhance efficiency or extend the reach of care. It refers to developing new technology, finding new applications for existing technology and new service models.
Innovation often creates value by improving the quality and efficiency of health services. Innovative and appropriate medical technology can free resources to provide better healthcare coverage to growing populations. Unfortunately, this concept is often not acknowledged and instead a short-term mindset, focused on immediate cost control, governs purchasing decisions.
Minimally invasive surgery – including robotic surgery, endoscopic surgery and laparoscopic surgery – has been one of the great success stories of innovation in medical technology. The use of advanced instruments allows surgeons to work through small incisions instead of opening up large areas of the body. Cameras, computers and instruments capable of very precise movements make these procedures possible. The benefits include less scarring, increased accuracy, lower risk of complications, shorter hospital stays, less pain and shorter recovery periods. (See below box on laparoscopic surgery).
Laparoscopes: One small device – a host of benefits
Laparoscopic surgery, performed with a laparoscope that enables the surgeon to visualise organs in the abdomen, avoids large incisions. This, and the use of fine instruments, reduces blood loss, tissue trauma, pain and discomfort. Patients need less analgesia and suffer fewer side-effects of analgesia. The rate of postoperative complications is generally lower.
Performing the operation within the body cavity avoids the cooling, drying, excessive handling and retraction of internal organs associated with “open” techniques. This may reduce postoperative peritoneal adhesions with the subsequent risk of bowel obstruction.
Laparoscopic surgery means less direct contact between surgeon and patient and therefore reduces the risk of infection passing between them.[4]
The recovery period is also shorter, lowering the risks of bone loss, muscle atrophy and urinary retention associated with lengthy bed rest. Other benefits of early mobilisation are lower rates of chest infection and deep vein thrombosis. Finally, patients prefer small scars and laparoscopic surgery reduce post-operative anxiety related to self-image.
It is critical that benefits of innovation are well understood and included in annual procurement planning frameworks to facilitate value-based procurement.
Medical technology and centralised procurement
In light of the special characteristics of medical technology, centralised, national tendering processes are rarely appropriate for purchasing in this field.
However, centralised tendering may be suitable for simple, standardised products required in large quantities across the health system. In such instances, quality remains paramount as does the efficiency of the procurement system, as system failure would carry heavy risks.
If centralised procurement is contemplated, the following factors need to be considered:
- An efficient and cost-effective central procurement process requires a single national database of all medical items, which is continuously updated in terms of stock levels at every healthcare facility. Without such a system, stock-outs or over-supply are inevitable and they carry the respective risks of poor patient care and unnecessary carrying costs.
- Product maintenance and servicing, and the training of staff in the use of technology are essential aspects of the procurement of many medical devices. If a centralised procurement system cannot incorporate these functions, it should not be used to purchase the relevant technology.
- Excessive use of centralised procurement risks creating monopsony (single-buyer) market conditions that can impede supplier competition and reduce the participation of small and medium-sized enterprises (SMEs) without a national footprint – clearly a disadvantage in a lower- and middle-income country (LMIC) setting such as exists in South Africa.
- Centralised tendering can severely limit therapeutic and diagnostic options across an entire healthcare system and may discourage innovation in standards of care.
- Even with a good information system, it may be difficult to strike a balance between avoiding device stock-outs that undermine healthcare delivery and maintaining an over-supply of devices, which leads to excessive carrying costs.
- The system must be capable of passing the “point of care test” – having the correct medical equipment, consumables and implants at the correct health establishment at the patient’s time of need.
- The system must be able to accommodate variations in demand from different districts, based on demographics, burden of disease and the capacity of healthcare providers. Similarly, it would need to accommodate the varying needs of districts for training in product use, maintenance and technical support.
- Finally, a centralised procurement system needs to remain sensitive to the value of competitive tendering in fostering innovation in medical technology and should reward features that bring new capabilities and improved options to the clinical pathway.
SAMED’s procurement principles
Transparent and equitable tendering
- All tendering should be conducted with transparent rules and open processes in which diverse products and services can compete on a level playing field, without prejudice regarding country of manufacture.
- Product categories for procurement purposes should be designed to create homogenous product groups that are truly comparable with respect to function, quality and clinical indication. The design of product categories should always be transparent and allow for public comment and clinical stakeholder feedback.
Overall greatest value
“Overall greatest value” methodology should generally be used when tendering for advanced medical technology. The economic impact and value of devices often depend on factors such as the longevity of the product, its quality in terms of performing clinical functions and the level of training, service and support provided by the supplier. Establishing overall greatest value requires a range of evaluation criteria which should be specified upfront in all tender documents.
Some tendering methods, such as internet-based “reverse auctions”, are typically unable to address these considerations and should not be applied to advanced medical technologies.
Market competition
Public tendering should be structured in a manner that encourages competition among potential suppliers and avoids the artificial restriction of the healthcare marketplace.
- Limits should be applied to the size and duration of tender contracts, so as not to create or perpetuate market monopolies. A ceiling should be set for the proportion of purchasing that may be bundled into a single tendering process.
- Multiple-source contracts are generally preferable in the medical technology sector, so that a diverse range of products and services is available for clinical use.
- In instances where product pricing and quality are similar, multiple awards should be made to ensure supply chain continuity.
Quality and innovation
- Tendering processes of all types should consider and adequately reward differences in product quality, innovative features and clinical value to patients. When cost is considered, it should include an understanding of lifetime patient costs and value to the patient and healthcare system.
- In order to be eligible to submit tenders, companies should be required to comply with the requirements of the South African Health Products Regulatory Authority (SAHPRA) and the regulations it administers. In respect of medical devices, the Medicines and Related Substances Control Amendment Act requires:
- Companies must currently be licensed by SAHPRA prior to manufacturing, wholesaling, distributing, and supplying medical devices.
- In future, SAHPRA certification of the quality of medical devices will be required.
Retaining localised tendering
Public sector hospitals and health districts should continue to have the authority to make autonomous product purchasing decisions in response to local needs. This delegation of authority may co-exist with more centralised purchasing, subject to the requirements for effective centralised procurement outlined above.
Harnessing relevant expertise
The tender process for medical technology should include individuals with relevant expertise and their advice should be incorporated into the design of tenders.
- Independent committees that include clinicians and medical device experts with no self-interest in relevant tenders can offer useful insight into quality-focused purchasing and help develop appropriate tender specifications.
- Where broader procurement policy is being developed, draft policy and/or legislation should not only undergo public consultation processes but those responsible should be responsive to the comments received from interested stakeholders, including patients, HCPs and manufacturers.
- All comments received should be summarised in a published document and the rationale given for decisions made in response to comments.
- Potential risks to quality, safety and patient access that are raised in comments should be addressed directly.
Legal provisions and international norms
All tenders should comply fully with the laws of the country, including those that seek to protect and promote competition in the market as this may have a considerable impact on the quality and innovative nature of products purchased.
Tendering should also be conducted in accordance with applicable international trade agreements, including those of the World Trade Organization (WTO).
Combatting corruption
Responsibility for curbing and combatting corruption vests both in tendering authorities and suppliers from the medical technology industry.
- The obligation of the public sector to abide by all relevant legal provisions has already been mentioned.
- A further safeguard would be a requirement for all suppliers to be signatories to the Medical Device Code of Ethical Marketing and Business Practice.
- Consideration should be given to using the open contracting data standard (see https://standard.open-contracting.org/latest/en/), which would minimise opportunity for corruption.
- New entrants to the market must be rigorously vetted and tender adjudicators should consider the time period over which a company has been manufacturing or supplying the type of product required.
- Tendering activities of various departments and municipalities should be monitored and evaluated on an ongoing basis to ensure that the above principles are positively supported.
- An independent appeals mechanism – such as a dedicated tribunal – should be established By National Treasury for companies that have reasonable grounds for contesting a tender award to lodge an appeal.
APPENDIX
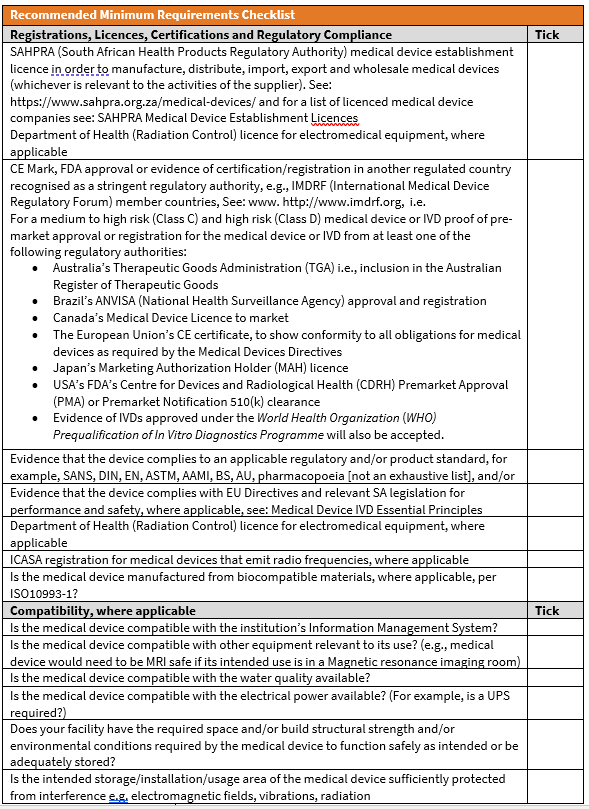
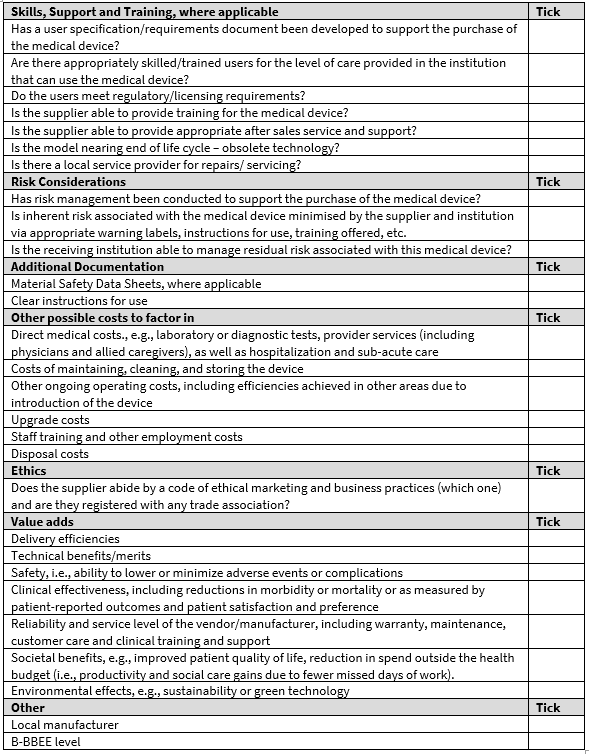
Guidance and checklist that can be used by medical technology procurement entities to ensure medical technology purchasing best practice
Five principles designed to help lower procurement costs and system risk, while enhancing overall performance:
- Evaluate the total cost of care: Less expensive products purchased to achieve immediate savings may generate greater costs in the long term. High-quality and innovative products that carry a higher initial procurement price can often generate improvements in patient care and reduced cost over alternative practices.
- Ensure clinical input: Cross-functional involvement of physicians, medical staff, administrators, data analysts, and other stakeholders in product selection ensure sufficient range of treatment options and guarantee that clinical needs are met.
- Use flexible contracts: Provisions for new product adoption help assure that contracts are flexible enough to provide rapid access to newly released advanced technologies.
- Encourage supplier diversity: Multiple-supplier contracts allow a larger number of suppliers into the market, which strengthens competition and ensures stability of supply. Less competition also reduces the procurer’s negotiation leverage in future rounds of purchasing, ultimately resulting in higher long-term procurement costs.
- Fair and transparent procurement processes: Minimise excessive bureaucracy and opportunity for corruption.
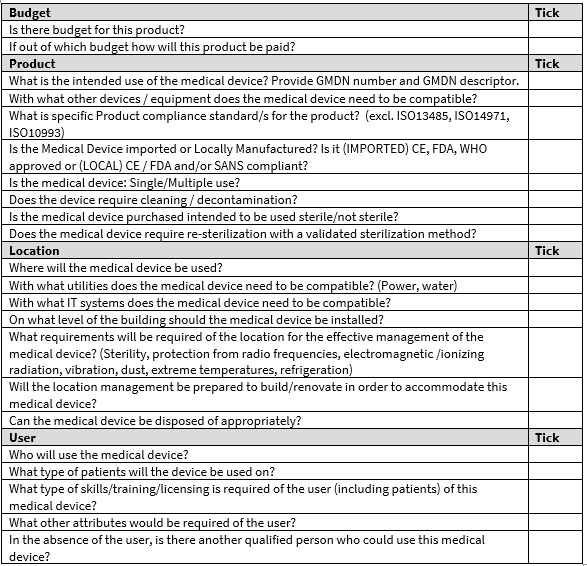
PURCHASING FRAMEWORK
A list of items that must be considered before concluding a purchasing decision:
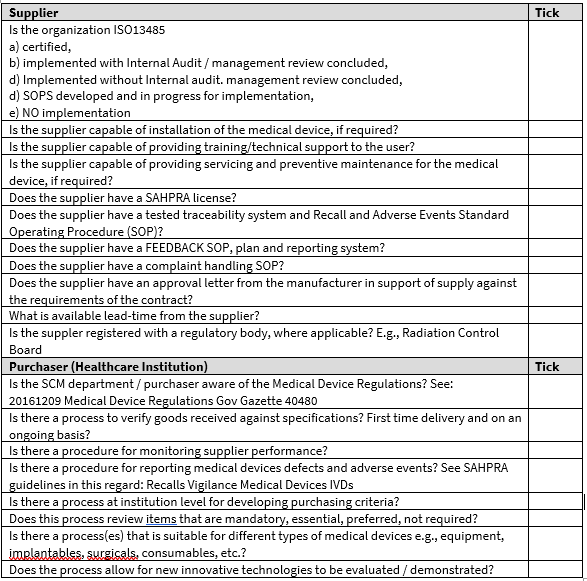
Source documents:
- Global Medical Technology Alliance (GMTA) position paper on Product Tendering, http://www.globalmedicaltechnologyalliance.org/papers/product-tendering.html
- Medical Technology Association of New Zealand (Mtanz) publication: Healthy Purchasing Decisions: A value-based approach Deloitte Report June 2019
- https://www.medtecheurope.org/wp-content/uploads/2018/01/2018_MTE_2pager_MTF-2018_project-overview_final.pdf
[1] Buying for a Better World U n i t e d N a t i o n s E n v i r o n m e n t P r o g r a m m e A Guide on Sustainable Procurement for the UN System. See page 19: https://www.ungm.org/Areas/Public/Downloads/BFABW_Final_web.pdf
[2] From value for money to value-based health services: a twenty-first century shift; 2020, World Health Organization https://www.who.int/choice/publications/vbhs.pdf?ua=1#:~:text=A%20health%20care%20system%20that,given%20the%20level%20of%20expenditure.
[3] Advamed. Good Practices for the Procurement of Innovative Medical Technology. See page 5: https://www.advamed.org/member-center/resource-library/good-practices-for-the-procurement-of-innovative-medical-technology/
[4] Journal of the Royal Society of Medicine: Does laparoscopic surgery spell the end of the open surgeon?
