SAMED Position on Health Technology Assessments: An exploratory paper towards the establishment of an independent Health Technology Assessment (HTA) Agency for South Africa
April 2023
Executive Summary
The South African Medical Technology Industry Association (SAMED) represents the interests of medical device, equipment, and in-vitro diagnostic (IVD) companies (collectively referred to as “medical technologies”) in South Africa, or medtech. This paper represents medtech industry position on the establishment and institutionalisation of HTA and a single HTA structure that is independent, transparent, and accountable to all its stakeholders and beneficiaries, however, requiring a different HTA approach to that of medicines.
Health Technology Assessment (HTA) is defined as a multidisciplinary process that uses explicit methods to determine the value of a health technology at different points in its lifecycle, to inform decision-making to promote an equitable, efficient, and high-quality health system. HTA in South Africa is underdeveloped and best described as supporting formulary decision-making and health policy decisions, rather than improving patient access.
In the private sector, new medical technologies are assessed by administrators to support reimbursement decisions by medical schemes, acting as primary gatekeepers for market access. A negative outcome of assessment translates into no reimbursement, non-approval on formularies and consequently access denied. Processes are fragmented and have variable lead times to decision-making. Methodologies focus is primarily on availability of randomised controlled trials, as opposed to best available evidence, and costs, rather than on cost effectiveness, value, and patient access.
HTA methods for medicines cannot be applied to medical technologies and needs to take cognisance of their unique characteristics, underpinned by continuous and iterative improvements, shorter product lifecycle, a relatively limited evidence base that may be outdated and/or irrelevant by the time an HTA is complete. There are specific aspects to consider in the methods for assessing medical technologies, including topic selection, scoping and communication with manufacturers, timing of assessments, dynamic development of new medical technologies, and relatively low levels of evidence.
A common thread throughout many policy documents is for the need for the establishment of institutionalised HTA, founded on the principles of independence, transparency, and participation (stakeholder engagement and consultation). There is, however, an absence of any empowering legislation for the formal establishment of an HTA agency, without which any recommendations will lack legitimacy and therefore enforceability.
This position paper discusses four major themes for establishing an independent HTA agency in South Africa, that may evolve into covering all health technologies, namely, good governance, structure, processes, and methodologies that are appropriate for medical technologies.
We explore the status of HTA internationally and locally, with a specific focus on medical technologies and the challenges associated with HTA of medical technologies and make recommendations for formalising and implementation of HTA in South Africa based on this experience.
A successful HTA organisation is premised on three core values of good governance: independence, transparency, and accountability, underpinned by relevant legislation that ensure processes and institutions produce results that meet the needs of society while making the best use of resources at their disposal.
An HTA structure in South Africa that achieves a segregation of functions, is rooted in the HTA value of independence from interest groups and political interference of any kind. The relationship of HTA to regulators (marketing authorisation) and decision making (coverage) bodies is critical as the interactions between these three core functions ultimately affects overall system performance. In an analysis of HTA across Europe, in most cases, HTA is separate from the regulator and coverage bodies. Similarly, within the HTA process itself and the three key tasks performed, i.e., assessment of therapeutic value, economic value, and appraisal – the degree of independence between these tasks also impacts the objectivity of the overall HTA outcome.
After studying HTA systems of different countries, in 2004, a Minister of Health appointed, multistakeholder, multisectoral committee made proposals regarding the scope and structure for HTA, guidelines for prioritisation of HTA activities, and the creation of a HTA Agency. Three possible options of the legal status of this body were considered., with terms of reference defined. Set standards for the process and methods were yet to be defined, with the organisation of HTA the focal point of design. The HTA model would include all stakeholders involved in health technologies, each with different skill sets, allowing existing HTA activities and processes currently functioning to continue, but providing their inputs into a central, but independent coordinating body. The HTA Agency would then, in turn, act as an advisory body to the relevant regulatory authority, and whose recommendations will then be used by coverage bodies e.g., NHI. The subsequently proposed NHI envisages the establishment of HTA within NHI, albeit as a transitional arrangement, which may imply that this will become an independent body at some stage as previously proposed.
A high quality HTA process should be efficient, ensure procedural justice, comprehensive, and communicated appropriately. HTA may be initiated by any stakeholder, wanting to assess new, redesigned, modified, or improved technologies, which are then classified and prioritized, conditional only passing safety standards (market authorization). Provision must be made for stakeholder participation and maximum transparency and must consider societal and institutional impact.
Standardized processes for medtech from the perspective of HTA institutions are not common, and therefore questions if a homogenous approach i.e., one size fits all approach should be used across all health technologies. Special characteristics of medtech raise additional challenges which require the HTA community to reflect on whether the current methods are adequate and deserve bespoke methods of assessment. While the typical five step HTA process may be fundamentally generic, may work seamlessly in the assessment of medicines, whereas when applied to medtech, need to consider design features that include innovative approaches for assessment, estimating and anticipating learning curves, use of observational data to assess comparative effectiveness, studying the diffusion and adoption and outcomes, and coverage with evidence development.
Evaluation pathways with explicit processes and improving methodologies for the evaluation of medtech, considering known challenges, are evolving in HTA. They are designed to identify, evaluate, and encourage adoption of new and innovative medtech, including, how it integrates into current clinical pathways and comparative effectiveness. Processes are relatively short with lead times strictly adhered to. Broad consultation is encouraged, including manufacturers, to identify strategies that can lead to sound solutions Clinician guidance may supplement available evidence and relevant gaps. Mechanisms for managed access are continuously being developed, with stakeholders engaged at relevant stages of the evaluation process. Process times should be managed consciously, as information changes rapidly, important to ensure that the process and methods remain relevant and focused on the best outcomes for patients.
Given the international learnings and the intentions as described within South African Policy documents referring to HTA, the medtech industry through SAMED supports such initiatives and makes proposals for an independent HTA organisation. Considering resource constraints, there is a strong case for leveraging off existing capacity and coordinating of such activities for a common purpose, primarily to reduce duplication of work, improve standardisation, reduce bias in appraisals, encourage transparency, increase credibility, improve levels of compliance and not least of all independence.
A system process archetype is recommended, where the functions and roles of regulator, HTA and coverage bodies (CB) be independent of one another, with the HTA function being independent of any bias from stakeholder influence and conflicts of interest.
An HTA process archetype is recommended that includes assessment of therapeutic and economic value and appraisal by an independent appraisal committee, envisaged as a multistakeholder group appointed by the relevant health technology unit of the HTA agency, including industry, medical practitioners, funders, and patients, elected by stakeholders, who will formulate their recommendations based on information received from the assessment/analysis phase.
The independence of the Appraisal Committee is essential and in terms of the composition thereof, should be represented by different stakeholders including academics, patient groups, industry etc. Stakeholders like SAMED should have the right to nominate a representative to sit on the Appraisal Committee (or a Dispute Committee in the case of a negative HTA outcome).
The proposed institutional structure is primarily an administrative function, with minimum permanent staff systematically scaling up as resources allow, to fulfil the term of reference (TORs) described, with the potential for conducting a limited amount of in-house research work. The majority of the HTA technical work is intended to be outsourced, operating within a set of established process and methodological guidelines. These are to be developed for each HT if and where necessary. Consideration can also be given to the function of environmental scanning, whether insourced or outsourced.
This is not a proposal for single HTA ‘agency’, for all technologies, performing all functions, which requires significant administrative overhead and cost, and the massive scale up of resources to be properly effective for the HTA of all qualifying technologies, which furthermore will take significant time to accrue.
This is a proposal for a smaller, government funded/public co-ordinating office that utilises and optimises public and private capacity efficiently. At the core of the HTA sub-system is the coordinating office as described, served by a medicine/vaccine technology unit on the one hand, and a medical technology unit on the other, each serviced by their own relevant external evaluation groups (public or private) and responsible for constituting appraisal committees representing relevant stakeholder groups. Terms of reference for each group will need to be created.
Advantages of such a structure recognises current capacity and potential partnerships, functions as a coordinator of internal and external activities, and enables evaluation centres to focus on technologies within their scope of expertise. Disadvantages may appear to stakeholders as not independent of the NDoH, thus legislative framework and governance arrangements are critical to ensure independence of expert committees and transparency of operations. To build and maintain credibility for HTA in South Africa, a fundamental set of principles are described.
SAMED proposes within this policy document that, after market authorisation by SAHPRA, any stakeholder, including suppliers, may initiate a submission for HTA for a new a new health technology. Escalation to independent HTA is contingent ONLY on the medical technology passing regulatory requirements. It is not uncommon, however, to conduct HTA in parallel to the regulatory process, where there is sufficient recognition of therapeutic and economic value. This may be determined in the planning phase, that will follow separate processes.
The HTA process is envisaged to follow the classic five step process, and this paper unpacks each of the functions, activities, and methods, underpinned by a set of key principles relevant to medical technologies, in a non-exhaustive list of each. It is expected that there may be many iterations of the development specific processes and methods for assessing medical technologies, subsequent to consensus being reached after engagement with relevant stakeholders.
A strategy is proposed for the institutionalisation of HTA in South Africa. This neither means, nor requires, the creation of a stand-alone fully capacitated HTA agency, but rather establishing accepted processes, norms, and standards, developing critical skills, experience, knowledge, and building effective working relationships between stakeholders to support the use of HTA. It ensures that HTA becomes part of the decision-making process in the health system, even if it is initially limited in its use to specific technologies i.e., innovative medical technologies.
Current activities in HTA should be recognised, integrated, and coordinated into the larger HTA sub system. Where the assessment of medical technologies and related procedures is multi factorial and complex, a “one-size-fits-all”, top down, implementation strategy is not adaptable nor practical, where there is a need to accommodate the differences in medical technologies and the unique operating contexts in which they are used. In this context, the medtech industry should be allowed to contribute to the development and implementation of HTA and its relevant processes and methods.
The foundations for institutionalising HTA are to audit existing HTA activities, capacity, and identify relevant stakeholders. Stakeholders, such as patient groups, policymakers, provider groups (hospitals and health care practitioners), academics, the healthcare industry, external partners/donors, and the public may be involved at several stages of HTA. The stakeholder analysis will help identify training needs some groups may have, as well as building a truly inclusive process. This is of particular importance to medtech, where clinical stakeholders help to identify and solve implementation issues unique to their hospital institutions, another stakeholder critical to medtech implementation.
Collectively, having well-trained and technical capacity with relevant skills in producing and using HTAs is key to HTA institutionalisation. Many observers cite the lack of capacity as one of the constraints on the development of HTA. This is also linked with a lack of skills.
SAMED proposes a critical first step would be to host an HTA summit that would include relevant stakeholders, with the aim to gain consensus on an acceptable and useful definitions of HTA, discuss the policy and legislative requirements for a national HTA agency or alternative mechanisms in South Africa. A strategy implementation framework is proposed for the institutionalisation of HTA in South Africa which parallels the process for introducing universal health coverage. This includes the mission, vision, major goals, key objectives, and goals for achieving key objectives.
In conclusion, SAMED strongly recommends a process archetype that delineates the functions and roles of regulator, HTA and coverage bodies and that these are independent of one another, with the HTA function specifically being independent of any bias from stakeholder influence and conflicts of interest. This archetype includes an independent appraisal committee, envisaged as an independent multistakeholder group.
In identifying different possible institutional arrangements for SA, we need to consider what will work best for this country, and whether this involves one or more units within the NDoH or an entirely independent body. There are trade-offs between those options, mainly on cost, links with other health agencies, transparency, and visibility. For instance, it might be cheaper to incorporate HTA within an existing department rather than setting up an entirely new department, but harder to ensure independence and transparency of decision-making.
It is expected to see greater convergence between sectors as NHI, or elements thereof, are implemented to achieve universal health care (UHC) for the entire population. In an environment of limited resources, HTA can play a pivotal role in informing decisions regarding access, utilisation, and value of health technologies. SAMED supports the building a sustainable and locally relevant HTA mechanism for priority setting that can support progress toward UHC.
This paper concludes with key principles that underpin the establishment of an independent HTA Agency for South Africa, the design of its structure and governance model, and medtech relevant processes and methods.
SAMED is a not-for-gain industry association established in 1985 and is committed to enabling a sustainable, ethical, and transformed medical technology industry that ensures patient access to medical technologies.
Over the years SAMEDs membership has grown significantly and includes 180+ multinationals, distributors, wholesalers and local manufacturers of medical devices, medical equipment, and in vitro diagnostics (IVDs) (collectively referred to as ‘medical technology’ or ‘medtech’).
Medical technology plays a vital role across the continuum of patient care and effective healthcare delivery (prevention, screening, monitoring, diagnosis, treatment, and rehabilitation). Medical technologies are highly variable in nature and include medical devices, medical equipment and IVDs. The definitions of “medical device” and “IVD” as included in the Medicines Act are as follows:

SAMED has been a long-time active participant and contributor to the discourse surrounding the health technology assessment (HTA) of medical technology.
This paper represents the SAMED position on the establishment and institutionalisation of HTA and a single HTA agency that is independent, transparent, and accountable to all its stakeholders and beneficiaries. This is against a backdrop of the origins, practices, and challenges of HTA in assessing medical technologies, the HTA policy landscape within South Africa and proposals embedded in the current NHI Bill and its recommendations to establish an HTA agency in South Africa.
Private and public stakeholders, themselves in different stages of HTA evolution, are positioning themselves as central to the establishment of such an agency. A recent example of this was the circulation mid 2021 of a proposed HTA Methods Guide by the Essential Drug Program (EDP), within the National Department of Health (NDoH) to all stakeholders for comment, with a view for adoption by a national agency and potentially used for all medical technologies.
While a significant step forward, which admirably aims to formalise and standardise HTA processes and methods, they are heavily biased towards medicines, failing to acknowledge the variety of medical technologies, their relative position in their life cycles and a need for bespoke methods for assessment. What is encouraging however, is that they do consider assessment beyond effectiveness and efficiency to include equity and other social values, while falling short of organisational considerations, critical to the appraisal of medical technologies.
The implication, however, is that the EDP may become the core of an HTA Agency in South Africa, and potentially, within the NHI, and likely experience the same challenges assessing medical technologies as has been the case in other countries, with subsequent difficulties with implementing recommendations.
The medtech industry requires a different HTA approach than that applied to medicines, with selection of technologies to be assessed made by all stakeholders, using methodologies and guidelines considering the peculiarities of this sector, with an option to allow generation of real-world data in the post-launch period via a collaborative multistakeholder model. In the early stages of development of institutionalised HTA, programs should prioritise on technologies that are innovative and address high unmet patient needs in disease areas where appropriate clinical and economic evidence has been, or can be, generated (e.g., innovative, transformative medical technologies).
Recommendations are made within this paper for appropriate governance, structure, processes and methods to be in place to facilitate independent, legitimate and relevant HTA of medical technology in South Africa and in so doing, eliminate divergent recommendations from multiple HTA processes, influenced by different priorities, in individual settings, in terms of both perceptions of benefit and value, and tools used to assess the HTA appraisal process. An independent co-ordinating HTA agency for South Africa “eliminates” multiple organisations doing different things arriving at different conclusions.
2. Current Status of HTA in South Africa for Medical Technology
Health Technology Assessment (HTA) is defined as a multidisciplinary process that uses explicit methods to determine the value of a health technology at different points in its lifecycle. The purpose is to inform decision-making to promote an equitable, efficient, and high-quality health system.(1)
HTA for medical technology lags in its’ journey in South Africa compared to the rest of world, in that although there may be Health Technology Assessment-like activities conducted, they are highly fragmented, variable, insufficiently transparent, not well understood, lack independence and are uncoordinated, i.e., no prioritisation or topic selection mechanism with limited stakeholder consultation. In the private funder sector, positive assessment decisions are not a guarantee for reimbursement across all medical scheme plans/options and negative decisions result in exclusion of access to the market and consequently patients. A medical scheme may decide on reimbursing for the richer plans i.e., no funding on lower plans. Another element to this is that the scheme may reimburse but if there are limits (prosthesis) or formularies, the medical technology component may be excluded.
This is contrary to international trends where HTA agencies have transparent review processes, independent appraisals by multi sectoral representative committees, and mandatory recommendations that are publicly available. (2) Local practice can best be described as supporting formulary decision-making, medical scheme clinical policies and cost cutting exercises, rather than improving patient access, with little differentiation in methods of assessment for different health technologies, i.e., use of by and large medicines related methods of assessment for medical technologies.
In the private sector, innovative medical technologies are subject to assessment of clinical and cost effectiveness by medical aid schemes (MAS) to inform decisions regarding inclusion in benefits packages and level of reimbursement. This may follow an in-house classification and price referencing process, where if a new class of medical technology (i.e., without direct comparator) and/or with an incremental cost increase this will be escalated to an HTA Information requirements normally include population (indication/s), intervention (technology description and related intervention, user and relevant training), comparators (local standard of care) and outcomes (clinical and economic evidence). There is neither provision, nor request for information of any ethical, legal, social and/or organizational impact; this may be volunteered by an applicant but not seriously considered by the medical aid scheme as relevant.
Application templates differ by medical aid scheme, which by design limits quality of information provided. Systematic research is still conducted internally by the medical aid scheme/reviewer organisation. Comparative evidence is preferred, ideally randomized controlled trials (RCTs), with clinical guidelines, where available. Grey and unpublished literature is excluded. Engagement with clinical stakeholders and industry is limited, with patient input often not considered. Final recommendations may be made anything from 6-24 months of application, without an opportunity to appeal within 12 months of notification of the decision and only if new evidence is available.
In this context the respective clinical policy unit CPU (doing the assessing) which falls within the administrator/managed care organisation make recommendations and the scheme (separate from the administrator) makes the decision to fund or not. Applicants may be provided the opportunity to engage with the assessment team, but not always guaranteed. In cases of uncertainty of evidence, a pilot may be entertained, but this is by exception. There is no prioritization process for HTA, nor any tracking mechanism. Assessment summaries are rarely publicly shared.
Assessment and subsequent reimbursement pathways may be different depending on whether the medical technology is a consumable/disposable, equipment, with or without a consumable/disposable component (e.g., a delivery/interface with patient), where associated with a specific intervention, and furthermore where the technology will be used e.g., hospital, ambulatory care, office based etc. These pathways may require inputs and active involvement by different stakeholders, who are inextricably linked with the appraisal process, adding to the complexities uniquely relevant to the assessments of medical technologies.
Private hospitals may become aware of the availability of the new technology, either via supplier and/or user, but are not typically involved in the HTA process until it has been approved, at which time it is introduced into the institution, who will conduct a hospital based HTA that considers relevant operational requirements, for example staffing, engineering requirements, facilities etc. The scope of HTA activities performed by medical aid schemes and hospitals is trending towards price benchmarking and assessment of safety, performance, and quality (which are regulatory and not health technology assessment aspects), irrespective of the international and local regulatory status of that medical technology.
In pursuit of universal health care (UHC) through the National Health Insurance (NHI) and the prominent role that HTA will have in informing decisions about access to medical technology under the NHI, it is necessary to be aware of the purpose and intent of HTA and how it has originated in different economies i.e., developing vs developed. HTA in developed countries is advanced, comprehensive, and well resourced, with a focus on improving patient access to health technology. In developing countries, it is conducted in a fragmented way as a cost cutting mechanism, with less emphasis on patient access.
Professional societies focus their evidence-based medicine (EBM) and HTA (to inform practice guidelines) activities on selected diagnoses and management of clinical conditions, and assessment of specific diagnostic tests, procedures, drugs, and medical technologies of interest to their members. Some medical technology suppliers conduct or commission HTAs on their own products, to support economic value dossiers for assessment.
HTA is internationally well defined(1) and the definition should not need further deliberation in South Africa. For purposes of system design and resource development, it is further differentiated into ‘micro’ and ‘macro’ HTA, focusing on the assessment of individual medical technologies and the effectiveness and efficiency of interventions within the whole health system, and informing the prioritization of health care services, respectively.(3)
HTA’s conducted in emerging markets, including South Africa, largely target new technologies that are seen as cost drivers, where the focus is on cost containment rather than identifying value. Thus, it appears that HTA is not being used in an effective way by these health care systems.(4) In South Africa medical technologies within an existing category/classification already approved for reimbursement, are increasingly being subjected to HTA, adding to lead times for introducing new alternatives and competitor medical technologies in the same class.
3. HTA Challenges for Medical Technology
Characterized by a high rate of innovation, medical technology undergoes continuous and iterative improvements, with shorter product lifecycles and investment recovery periods than for pharmaceuticals. Evidence may be out of date and/or irrelevant by the time an HTA is complete, with new iterations of the technology already used in the marketplace. HTAs should be designed to reflect these attributes and limitations to the evidence base. Medical technology differs in several respects to that of pharmaceuticals.
HTA methods for pharmaceuticals cannot be applied to medical technology and should consider medical technology as complex interventions, requiring use of appropriate approaches that recognize and allow for the distinct characteristics of medical technology. Further challenges specific to assessment may be experienced from a structural, procedural, methodological, and regulatory perspective. (5)
These differences influence how comparative effectiveness research can be performed and require modifications in assessment methods. A high-risk medical technology may be combined with surgical procedures and are part of complex interventions. HTA methods, structures, and processes need to be adapted to allow for the patients’ rapid access to effective and safe medical technology. There are specific aspects to consider in the methods for assessing medical technology, including topic selection, scoping and communication with manufacturers, timing of assessments, dynamic development of technology, and low levels of evidence. (6)
HTA agencies seldom differentiate between methods and processes used for medicines and medical technologies.(7)(8) Methods guides focus on medicines, although some agencies have created specific guides and dedicated relevant pathways for medical technology. Approaches to overcoming these challenges include far greater interaction with relevant stakeholders, use of horizon scanning programs, post marketing surveillance, coverage with evidence development, addressing missing data and facilitating the conduct of research studies/set up of a register. (9)
4. Legal Frameworks Supporting HTA in South Africa
Regulation of health technologies falls within the mandate of the South African Health Products Regulatory Authority (SAHPRA), whereas HTA activities, are applied to private sector reimbursement decisions. An HTA system that independent of a regulatory function enables decisions about resource allocation to be separated from decisions about safety, thus facilitating accountable and clear decision-making systems. A well-functioning SAHPRA will be critical to the success of HTA in South Africa.
HTA in South Africa has been an evolving discussion for over two decades, featured in several policy documents, the NHI Bill, the Health Market Inquiry and the Presidential Health Compact. In recognition of the need for developing skills and capacity, reference is made to the establishment of scientific organisations who are positioned to guide and facilitate such training of skills required for conducting the technical elements of HTA as well as providing objective discussion forums for all stakeholders across the health sector.
A common thread throughout these documents is the need for the establishment of institutionalised HTA, founded on the principles of independence, transparency, and participation (stakeholder engagement and consultation). There is an absence of empowering legislation for the formal establishment of an HTA agency, without which recommendations will lack legitimacy and therefore enforceability.
5. HTA Governance models and Structural Archetypes
Common lessons learned of the challenges of HTA, and its successful implementation, have been extensively researched and reported.(11) (12) These lessons should be considered in the design and development of HTA in South Africa. Three core values of governance, that is, independence, transparency and accountability are found to have major influence over the most successful HTA organisations.(13) Organisation of HTA requires the broadest possible scope in its appraisal of all evidence and ensure its processes are designed as such.(12)
A WHO Global Survey of HTA Agencies provides insight into impediments and opportunities for developing an appropriate HTA model for South Africa.(14) Developing a system to use HTA requires not only methodological capacity, but also governance structures, including legislation, with clear lines of accountability for decision-making. Political support and leadership are essential. There is a need to develop a structure that best meets local needs, and this may or may not be a single independent organization. It was noted that developing partnerships with academic institutions was a useful strategy.(15)
An HTA structure in South Africa that achieves a segregation of functions, is rooted in the HTA value of independence from interest groups and political interference of any kind.(16)
In an analysis of the pathways for regulatory, HTA and coverage decisions across Europe was conducted using a mapping methodology to illustrate HTA organisation relationships and integration with regulators (those providing market authorisation for medical technologies) and coverage bodies (those making funding decisions). Following gathering of information on core functions and activities, their similarities and differences were used to create two groups of taxonomies for the health systems.
The first taxonomic grouping (Figure 1) is based on the position of a national HTA agency (HTA), if present, in relation regulatory (REG) and the coverage body (CB), and where these functions are combined or independent of one another.
| Figure 1: System process taxonomy | |
| S1: Regulatory, HTA and coverage body functions are performed by separate agencies.
S2: Regulatory and HTA functions are performed by a single agency and the coverage body functions are independent. S3: HTA and coverage body functions are performed by a single agency with the regulatory function performed independently. S4: Regulatory, HTA and coverage body functions are all performed within a single agency. S5: No HTA is performed within the national regulatory to reimbursement system. |
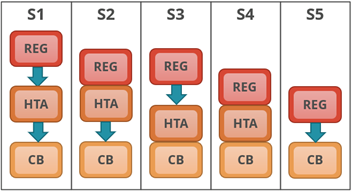 |
The other grouping (Figure 2) is based on the three key tasks performed by the HTA agency and relative positions of each, if performed, within the HTA agency: therapeutic value (TV); economic value (EV); and appraisal (AP), and, also, whether performed within the same agency or independently of each other or not.
| Figure 2: HTA Process Taxonomy | |
| H1: Therapeutic value is assessed prior to independent appraisal.
H2: Therapeutic value assessment is conducted within the same agency as economic evaluation, but the appraisal is performed independently, usually by health professionals rather than civil servants. H3: the therapeutic value assessment, economic evaluation and appraisal are performed within the same agency. H4: Appraisal is conducted using information from an external HTA report or by considering the coverage decisions of reference countries. |
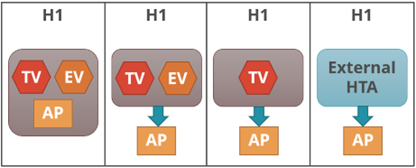 |
The two taxonomic sets were cross-referenced to help identify practical archetype groupings of HTA agencies (Figure 3). Each group indicates an archetype of HTA agencies that have similar positions, in their respective coverage systems, and perform comparable key HTA tasks. Ten distinct HTA archetypes were identified. In most cases HTA is separate from the regulator, and in about half of these perform the appraisal as well. The interactions between these three core functions i.e., the regulator, HTA and coverage body (payer) can ultimately affect overall system performance. (17)
| Figure 3: HTA Archetypes | |
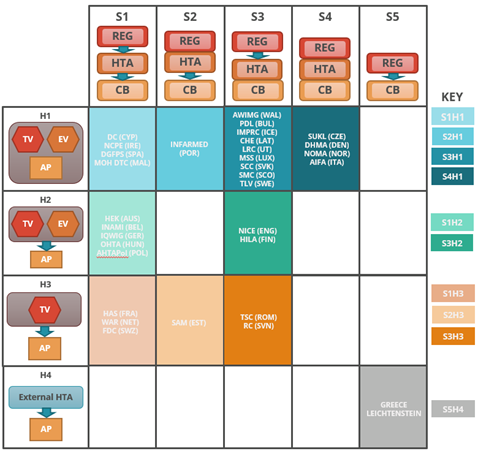 |
This cross-reference grid shows to which system and HTA process taxonomy group each HTA agency, and its respective national system, have been allocated.
The ten archetype groups are listed by their abbreviations in the archetype key and each group’s position is represented on the grid by colour. Boxes with no agencies have been left blank. |
Most countries, including South Africa, have a two-stage process for assessing medical technologies. The first relates to regulatory / marketing authorisation (product registration), including the appraisal of safety, performance, and quality, conducted by SAHPRA. The second uses separate processes and HTA-like activities to make recommendations for reimbursement. HTA requires a completely different set of skills and type of evidence needed for review and appraisal and therefore it is critical to segregate these functions.(18)
HTA activities in South Africa are currently fragmented. There are a variety of skill sets that exist across the sector within academia, funders, national and provincial departments, hospitals, consultants, and industry, although mostly residing with persons involved in clinical research, epidemiology, and health economics.
A multistakeholder, multisectoral Interim Steering Committee for HTA (ISC-HTA), appointed by the Minister of Health in 2004, after studying the HTA systems of different countries, made proposals regarding the scope and structure for HTA, guidelines for prioritisation of HTA activities, and the creation of an HTA Agency. Three possible options of the legal status of this body were considered: a legal person, a coordinating/ collaborative office of current activities, or expanding the mandate of an existing body. The terms of reference for the establishment of a permanent HTA structure were defined. The status quo remains, however, where there continue to be entities in the public and private sectors that perform different HTA activities. It is therefore recommended that focus be given to setting standardised methods and processes.
A proposed HTA model should include all impacted stakeholders making inputs into a central, but independent coordinating body. The HTA Agency should act as an advisory body to the relevant regulatory authority whose recommendations will then be considered by coverage/ reimbursement bodies e.g., the NHI fund and medical aid schemes.
The proposed NHI envisages the establishment of HTA within NHI, albeit as a transitional arrangement, which may imply that this will become an independent body at some stage. It is proposed that an interim Ministerial Advisory Committee on HTA (MAC-HTA) be constituted to oversee the organisation of such an entity, reporting to the MoH and advising the NHI Benefits Advisory Committee (BAC). The latest Bill is not explicit regarding the independence nor permanency of an HTA agency within the structures of the NHI.
6. Processes and Methodologies for HTA of Medical Technology
Key issues that must be considered in the design of an HTA Agency and process that is both workable and sustainable, relate to cost and practical elements, optimal utilisation of available resources and efficiencies. These are based on international HTA experience that has proven that it can be extremely expensive, with the budgets of HTA agencies literally running into millions of dollars, and that the turnaround times on research projects are quite long, ranging from twelve to eighteen months per project.
A high quality HTA process should: be efficient (prioritisation; topic selection; avoid duplication), ensure procedural justice, comprehensive (consider all core domains as per the EUNetHTA model (19), and communicated appropriately. It may be triggered by an innovative medical technology, re-evaluation of an existing medical technology or redesign, significant improvement, or modification of an existing medical technology. It may be initiated by any stakeholder. Escalation to HTA is conditional on passing regulatory requirements (market authorisation). There must be provision for stakeholder participation and maximum transparency.
There are opportunities for South Africa to leverage off work that has been done globally on medical technology appropriate HTA processes and methods, for example The National Institute for Health and Clinical Excellence (NICE), the Medical Services Advisory Committee (MSAC), and the Canadian Agency for Drugs and Technology in Health (CADTH). One key to success within a limited budget environment is to make optimal use of existing constrained capacity and resources by considering HTA decisions made by other agencies taking into account the local context.
A detailed analysis on common procedures of HTA institutions in the context of defining relevant outcome measures for the assessment of medical technology shows that standardized procedures for medical technology from the perspective of HTA institutions are not common. This leads to the question if a homogenous approach should be implemented in the field of HTA on medical technologies?(20) Special characteristics of devices raise additional challenges which require the HTA community to reflect on whether the current methods are adequate.(21)
HTA processes are fundamentally generic, but should broadly include five major steps in the process, and as HTA cannot be seen as a linear process each element should provide a feedback loop into the other for iterative development of the process and review of recommendations:
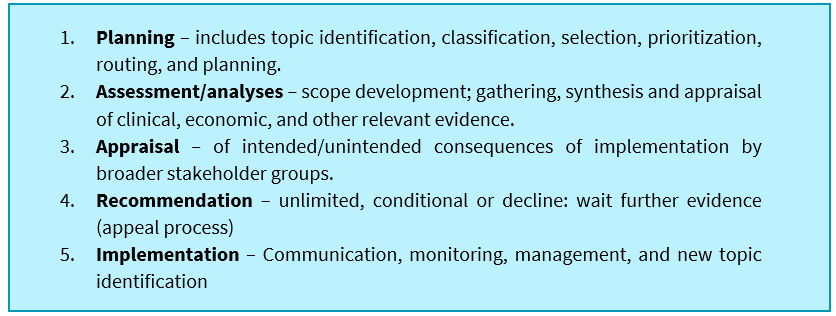
These steps all feature in processes proposed previously by the ISC-HTA, are currently mostly used in South Africa by medical aid schemes, albeit in varying degrees of depth and within the Essential Drug Program (EDP). Medical schemes in South Africa do not explicitly consider other appraisal domains such as ethics, legal, social, and organisational aspects (ELSO).
Key process design features important to the research and assessment of medical technology should include:
- Developing innovative approaches for the assessment of medical technology
- Estimating learning curves for medical technology
- Use of observational data in assessing the comparative effectiveness of medical technology
- Studying the diffusion of medical technology
- Coverage with evidence development for medical technology.(22)
Internationally HTA agencies, such as NICE, MSAC, CADTH and the Singapore Agency for Care Effectiveness (ACE), as an emerging economy, recognise the need for specific evaluation pathways for medical technology and have explicit processes and methods guides. These are designed to identify, evaluate, and encourage adoption of new and innovative medical technologies. Medical Technology suppliers are invited to submit evidence and indication of how the technology integrates into current clinical pathways and how it performs in relation to current best practice. Lead times can be an approximate six weeks and are strictly adhered to.
These programs encourage broad consultation, particularly with expert advisors to complement available evidence. They may also include mechanisms to facilitate patients’ early access to innovative medical technology, early in its life cycle, with continuous engagement with relevant stakeholders at relevant stages of the evaluation process. the European Network for Health Technology Assessment (EUnetHTA), has launched a series of research initiatives to develop a methodological framework for HTA of therapeutic medical devices.
During conducting an HTA, gaps in information may be identified that can prevent or delay a timely and accurate assessment, which in turn can delay patient access to important technologies. When this occurs, the organization conducting an HTA should consult with stakeholders – including suppliers – to identify strategies that can lead to sound solutions. These solutions may include conditional recommendations, post-market monitoring and cost-sharing with industry to develop additional data. Also, because available information on health technologies may change over time, assessments should be revised periodically. This is important to ensure that they remain relevant and focused on the best outcomes for patients.
Many new medical technologies fall short in their assessment in South Africa due to a perceived lack of evidence. Availability of scientific evidence for medical technologies is substantially lower than for medicines, not least of all ethical issues associated with conducting randomised controlled trials, iterative changes and/or upgrades, learning curves and operator experience, selection of endpoints etc. Assessment bodies that inform coverage decisions persist with using medicines methodologies, the outcomes are predictably negative. There are many projects underway, aiming to improve methods of evaluation of medical technologies, considering the known challenges.(23)
In South Africa, as with other relatively late HTA adopter countries, medical technology suppliers should be able to use reported HTA recommendations from other countries where the medical technology is in use. (24)
Key success factors for developing an HTA process should include:

Methods in South Africa typically follow a classic research question development (PICO) approach, with an assessment of the population (P) that will be treated, the intervention (I) and the technology that is used, the comparator (C) relative to the standard of care in a local context and clinical and economic outcomes (O). They mostly ignore or apply less importance to the ELSO domains.
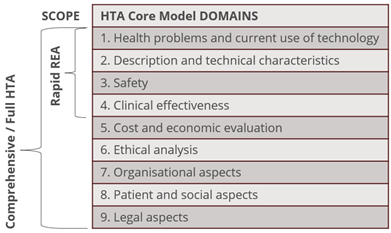
The EUnetHTA HTA Core Model®(19) (Figure 4) is an example of a methodological framework for collaborative production and sharing of HTA information whose aim is to enable international collaboration in producing HTA information and efficient sharing of the results so that redundant overlapping work in different countries and regions can be avoided. It defines the content elements to be considered in an HTA, enabling standardized reporting, providing a common framework for the production, presentation, and use of HTA. Information is organized similarly to what is practiced locally using the PICO approach, but explicitly calls for explicit inclusion of the ELSO domains that make up nine assessment elements (domains) of the model. Inclusion/exclusion of an element into/from the model does not make it unimportant, insignificant, or not otherwise worth considering in an HTA.
Rapid evidence assessments (REA) are intended for assessments within a limited time frame, covering only the first four domains, and may assess a new technology recently introduced to the market, or (re)assess a technology for a new indication or when new relevant data are available. This approach should be a pragmatic alternative pathway for many MDs.
Figure 4: EUnetHTA Core Model
7. A proposed HTA structure for South Africa
In the decision-making roles for market access for new medical technologies in South Africa, the medtech industry is recommending a system process archetype, where the functions and roles of regulator, HTA and coverage bodies (CB) be independent of one another, with the HTA function being independent of any bias from stakeholder influence and conflicts of interest.
An HTA process archetype is recommended that includes assessment of therapeutic and economic value and appraisal by an independent appraisal committee, envisaged as a multistakeholder group appointed by the relevant health technology unit of the HTA agency, including industry, medical practitioners, funders, and patients, elected by stakeholders, who will formulate their recommendations based on information received from the assessment/analysis phase.
The proposed archetype may be represented by the S1H2 archetype as described above, which may offer the most independent option for HTA making decision-making less susceptible to bias. This archetype implies that the Regulator is separate from the HTA Agency and that the Coverage Body and final decision maker is also a separate entity. Furthermore, under this archetype, the Therapeutic Value and Economic Value assessments may be conducted separately, but not to suggest that these may not be performed by the same unit, as implied in Archetype S1H1. in one unit, but the appraisal of their findings should still be performed by an independent Appraisal Committee which is multi-representative.
The independence of the Appraisal Committee is essential and in terms of the composition thereof, should be represented by different stakeholders including academics, patient groups, industry etc. Stakeholders like SAMED should have the right to nominate a representative to sit on the Appraisal Committee (or a Dispute Committee in the case of a negative HTA outcome).
Models of HTA differs between countries, with some finding it easier to create a clearly separate organisation providing independent assessments and guidance. Others have chosen to rely on an institutionalised HTA framework that is composed of multiple organisations, with clearly defined roles and responsibilities.
Given the above analysis and description of current and proposed structures and activities across different policy documents and organisations referring to HTA, there is still significant fragmentation, and a need to improve coordination of activities and practice.
Based on international experience, and the evolution of HTA Agencies in other markets, where HTA activities occur in the absence of any coordination and particularly in resource constrained developing countries such as South Africa, there is a strong case for leveraging off existing capacity and coordinating all such activities for a common purpose. Such coordination will reduce duplication of work, improve standardisation, reduce bias in appraisals, encourage transparency, increase credibility, and improve levels of compliance.
The objective of the proposed structure is to keep it as an administrative function, keeping appointment of permanent staff to a minimum, scaling up to fulfil the term of reference (TORs), with the potential for conducting a limited amount of in-house research work. The majority of the HTA work may be outsourced, operating within a set of established process and methodological guidelines, to be developed for each medical technology if and where necessary. Consideration can also be given to the function of environmental scanning, whether insourced or outsourced.
This model emulates Australian and Canadian models and is being adopted in developing markets systematically within the means of that country’s economy. This allows for the desired transparency through stakeholder participation and involvement.
Proposing an independent HTA ‘agency’, performing all relevant HTA functions, requires significant administrative overhead and cost. A further requirement would be the massive scale up of resources to be properly effective. This is not to suggest that private entities, along with public, may not be subcontracted and/or co-opted by a smaller government funded/public co-ordinating office.
Given the evolution of HTA in South Africa, and where we are in its lifecycle, the following model is proposed in Figure 5:
Figure 5: Health Technology Assessment Ecosystem
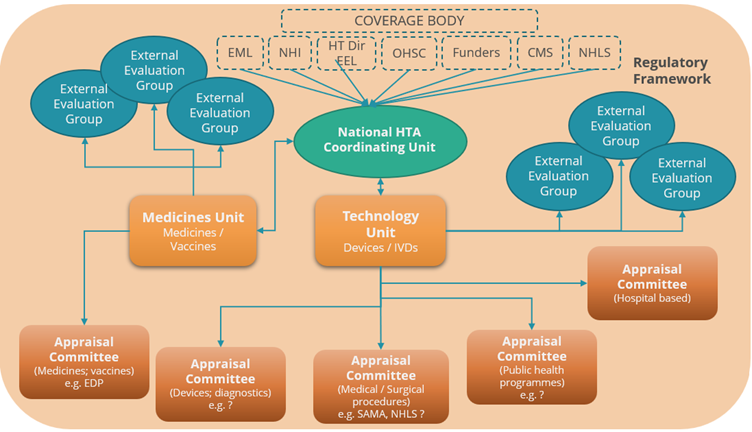
- A National Coordinating HTA Coordinating Office: An HTA ‘entry point’ for coordinating HTA’s requested by “coverage/decision making bodies” or any stakeholder within public or private sectors. Responsible for regulatory/legislative reforms/updates, promulgating policies; coordinating development and revision of clinical treatment guidelines; managing the appointment of expert committees; supporting review/appeal procedures; managing consultations and stakeholder engagement on matters of policy and process. The coordinating office could also be expanded to manage pricing, price negotiations and risk sharing arrangements.
- A Medicines HTA Unit, responsible for HTA for health products including medicines, vaccines, developing, and updating methods, managing, and providing support to relevant expert committees, contracting external academic groups to provide additional analytical expertise.
- A Technology HTA Unit, responsible for HTA of medical technologies developing, and updating methods, managing, and providing secretariat support to relevant expert committees, contracting external academic groups to provide additional analytical expertise.
- Contracted external academic/external evaluation groups, providing additional capacity with skilled resources in analysis of (clinical and economic) evidence, either reviewing submitted application dossiers or identifying and evaluating new evidence.
- Evidence appraisal via a series of independent expert appraisal committees each focusing on different technical areas, e.g., medicines/vaccines; diagnostics and devices; medical/surgical procedures; public health programs etc.
- An underlying regulatory framework setting out governance arrangements, appeal/ review rights and processes; articulating the composition, role, and responsibilities of the independent advisory committees (terms of reference) and the status of committee recommendations; and defining the scope and limits of the authority of the Minister of Health as the final decision maker.
Terms of Reference for each relevant group will need to be created.
Advantages of this model
- Explicitly recognizes external HTA expertise to complement limited resources (e.g., within the NDoH)
- Performs a coordination function of both internal and external activities.
- Enables external evaluation resources to focus on technologies reflecting areas of specific interest or expertise.
Disadvantages of this model
- May appear to stakeholders as not independent or at ‘arm’s length’ from NDoH, thus legislative framework and governance arrangements are critical to ensure independence of expert committees and transparency of operations (this has been seen to work with the SAMRC, a public entity).
To build and maintain credibility for Health Technology Assessment in South Africa the following principles should be adhered to:
- Should ideally be established by an act of parliament and/or current relevant empowering legislation.
- The independence of the agency must be safeguarded and unbiased, both in terms of its funding and its assessments.
- It must be objective and base its findings on ALL factual available evidence.
- The processes that are followed must be fully transparent.
- It must be effective and efficient, with time limits set and strictly adhered to.
- There must be a provision for appeals and review (of existing technology)
The outcomes/recommendations of the HTA should be:
- YES, unrestricted access.
- NO
- YES, with conditions.
Outcomes should be articulated clearly and unambiguously. It must be able to facilitate the implementation of its findings and recommendations.
Proposed Process and Methods for HTA for Medical Technology
SAHPRA is expected to fulfil its mandate to authorise medical technology entering the market that are of proven safety, quality, and performance.
Only after market authorisation by SAHPRA, any stakeholder, including suppliers, may initiate a submission for HTA for a new medical technology. Escalation to independent HTA is contingent ONLY on the medical technology passing regulatory requirements. It is not uncommon, however, to conduct HTA in parallel to the regulatory process, where there is sufficient recognition of therapeutic and economic value. This may be determined in the planning phase, that will follow separate processes.
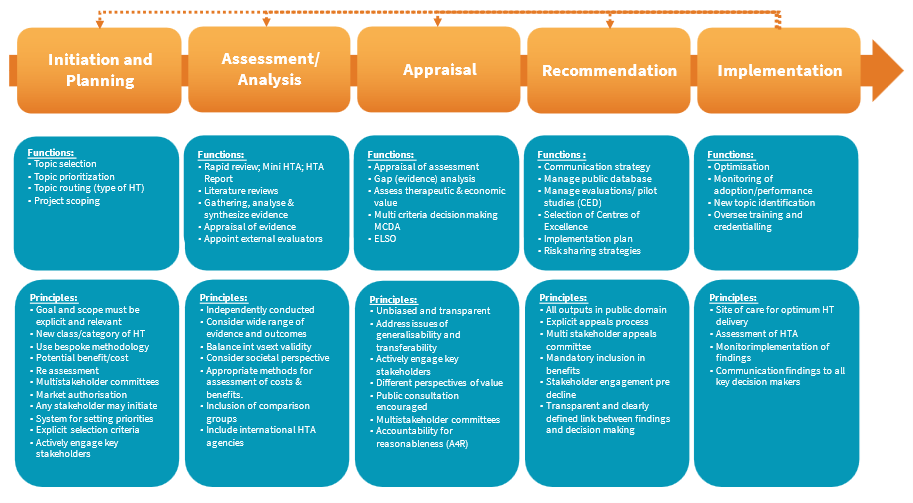
The HTA process diagram (Figure 6) represents a classic five step approach to HTA, ideally designed as a dynamic process, represented here by feedback loops providing for continuous review. Common functions of each phase are listed, underpinned by relevant functions and major principles that need to be considered in process design. This proposed framework provides a non-exhaustive list of functions and principles per phase, to be unpacked and refined after stakeholder engagement in the development process.
On entering the initiation and planning phase will, through project scoping and analysis of type of medical technology, provide guidance to applicants’ regard information requirements, and the assessment pathway that it may follow, i.e., either rapid review, mini-HTA or full-HTA, described later.
Specific selection criterion are required to determine what qualifies for HTA, avoiding unnecessary pressure on the system through arbitrary escalation of any and all medical technologies. Only new classes of medical technology should be selected for HTA, using, for example, relative risk (class), adoption, clinical need, and cost as part of a set of primary criteria. The GMDN classification tool may be used as a screening mechanism for selection. Prioritisation may be determined by the multistakeholder committee based on impact on burden of disease, unmet clinical need and potential value offered. Type of medical technology will influence scoping of the topic and determine the type of assessment required.
Given the diversity and variable complexities of medical technologies, a three-tiered process could be adopted for HTA routing to expedite a recommendation and avoid unnecessary delays to patient access and inappropriate use of resources. The EUnetHTA Core Model has been described above as a standardised framework for HTA reporting as a possible model, or facsimiles thereof, for adoption.
Within the assessment phase of the process, and the need for alternative HTA reporting options for medical technologies, the International Network of Agencies for Health Technology Assessment (INAHTA) has developed three types of HTA assessment strategies and/or pathways (25), adoptable in South Africa, described as Rapid Reviews, Mini HTA’s and Full HTA’s.
The scope of these strategies has yet to be fully understood for relevance to the SA context, the terms of reference and scope of devices defined for each, with possible different and new pathways developed for different medical technologies, based on the output of a stakeholder engagement process.
The assessment and analysis phase of the HTA process will collate and synthesise all relevant evidence that befits a comprehensive HTA, and based on pre-determined routing, will be referred to the relevant external evaluation groups.
The synthesised assessment and analysis (including appraisal of synthetised evidence) is referred to the appraisal phase via an external appraisal committee, will consider therapeutic and economic value, including appraisal of all other HTA domains, including ELSO where applicable.
The HTA process will yield a recommendation based on all the evidence before it, after wide stakeholder consultation. It may determine a cost effectiveness threshold but not recommend a price for that technology as HTA should not assume the role of a pricing authority. The HTA Agency will formulate an implementation plan and communication strategy based on the relevant recommendations received.
FIGURE 6: HTA Process for Medical Technologies
8. A Strategy for setting up HTA in South Africa
Institutionalising HTA requires establishing accepted processes, norms, and standards, developing critical skills, experience, knowledge, and building effective working relationships between stakeholders to support the use of HTA. It ensures that HTA becomes part of the decision-making process in the health system, even if it is initially limited in its use to specific HT’s.
On the other hand, where the assessment of MDs and related services is multi factorial and complex, a “one-size-fits-all”, top down, implementation strategy is not adaptable nor practical, where there is a need to accommodate the differences in HTs and the unique operating contexts in which they are used, as has been well described here and in many articles. In this context, allow the MD community to contribute to the development and implementation of HTA and its relevant processes and methods.
The foundations for institutionalising HTA are to audit existing HTA activities, identify which stakeholders need to be involved and what is the existing capacity, bearing in mind that HTA may go by another name e.g., evidence-based medicine, cost effectiveness analysis, clinical/health policy/guidelines development etc.
A stakeholder analysis is the process of gathering information to determine whose interests should be considered as part of laying the groundwork for introducing HTA, to identify who might support or block the process and develop strategies to mitigate the effects of opposition.
Stakeholders, such as patient groups, policymakers, provider groups (hospitals and doctors), academics, the healthcare industry, external partners/donors, and the public may be involved at several stages of HTA. Identifying relevant stakeholders will help identify training needs some groups may have, as well as building a truly inclusive process. More specifically, and closely linked to MDs, clinical stakeholders such as doctors assume the role of clinical champions to identify and solve implementation issues unique to their institutions, another stakeholder critical to MD implementation. A proper implementation team at the healthcare institutional level, comprising people with the necessary skillsets and authority to drive multifaceted changes, should be established to solve organizational problems.
Collectively, having well-trained and technical capacity with relevant skills in producing and using HTAs is key to HTA institutionalisation. Many observers cite the lack of capacity as one of the constraints on the development of HTA. This is also linked with a lack of skills.
A South African Health Review (SAHR) review of HTA in South Africa proposes that, for a unified vision for HTA in SA, an implementation process could include defining and aligning understanding of HTA through broad stakeholder engagement; align policies with NHI; harmonise legislation and policy; legislate amendments in Parliament; and implementation. A critical first step would be to host an HTA summit that would include relevant stakeholders, with the aim to gain consensus on an acceptable and useful definitions of HTA, discuss the policy and legislative requirements for a national HTA agency or alternative mechanisms in South Africa.(10)
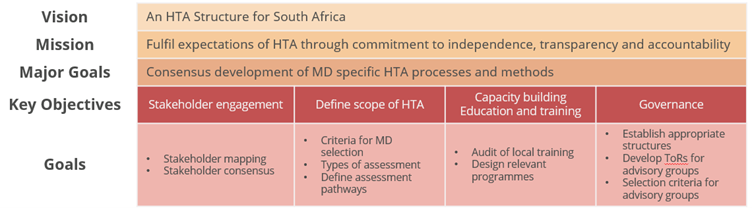
The medtech industry proposes the following strategy framework (Figure 7) for the institutionalisation of HTA in South Africa which parallels the process for introducing universal health coverage (i.e., NHI), typical of lower to middle income countries (LMIC), underpinned by a commitment to establish a political framework to promote (enforce, facilitate) HTA uptake and establish structures for timely, efficient, and good quality provision of HTA information.(26)
Figure 7: Strategy Roadmap – Health Technology Assessment
In identifying different possible institutional arrangements for SA, we need to consider what will work best for this country, and whether this involves one or more units within the NDoH or an entirely independent body. There are trade-offs between those options, mainly on cost, links with other health agencies, transparency, and visibility. For instance, it might be cheaper to incorporate HTA within an existing department rather than setting up an entirely new department, but harder to ensure independence and transparency of decision-making.(27)
The South African healthcare environment is currently considered a two-tiered system with diverse levels of care and access (public/private sectors) resulting in a highly fragmented system, not least of all in the HTA field. The future is expected to see greater convergence between sectors as NHI, or elements thereof, are implemented to achieve universal health care (UHC) for the entire population. In an environment of limited resources, HTA can play a pivotal role in informing decisions regarding access, utilisation, and value of health technologies. SAMED supports the building of a sustainable and locally relevant HTA mechanism for priority setting that can support progress toward UHC.
The establishment of an independent HTA Agency for South Africa, the design of its structure and governance model, and medical technology relevant processes and methods should be underpinned by the following principles:
- To avoid potential for overwhelming the HTA Agency, and complicating prioritization, selection of medical technologies for HTA should be limited to, for example, those falling into a new class of technology, where the impact of the technology may be significant and/or where there may a substantial difference in incremental cost and budget impact associated with introducing the new medical technology.
- Data reflecting epidemiological, resource and technology cost data should be made available, at no cost, by stakeholders e.g., claims data, registry data, public health facilities and private hospital data to inform an economic analysis and/or by the HTA Agency appointed external evaluation group conducting the analysis; sources of data should be transparent and verifiable.
- Align current and/or new legislation and policy requirements for HTA implementation, to ensure legitimacy of outputs and compliance, and determine if the empowering feature for HTA should fall under existing legislation (e.g., National Health Act) or a new set of regulations.
- Adopt a decentralized HTA structure, independent of health product regulator and coverage bodies, utilizing existing skills sets, both public and private, with a small coordinating office overseeing the activities of several sub-committees with definitive mandates.
- Independence of the HTA Agency is sacrosanct, and to ensure this requires a Board made up by health sector representatives, each nominated by relevant stakeholders, with CEO elected by the board, responsible to the Minister of Health.
- Ensure inclusiveness through maximum participation of all stakeholders, including government departments such as Finance, Trade and Industry, Science and Technology and Social Development, along with Health, who should have input in a functioning HTA Agency.
- From a health system process perspective, the health products regulator (SAHPRA) should remain separated, and as there are multiple downstream users of HTA representing coverage bodies/making decisions for their own constituents, the coverage body function should remain separate as well.
- Recommendations of the HTA agency should be mandatory, with stakeholders making provision for inclusion in guidelines and protocols, providing relevant benefits and coverage; negative recommendations should not be exclusionary, i.e., these HTs will still be available for use.
- A clear, fair, and transparent dispute resolution should be in place in the case of a negative recommendation of an HT, with terms of reference explicit and public.
- As the outputs of the HTA agency are for the benefit of the entire population, operating costs should be funded between government and less from manufacturer applications. Fees related to submission of dossiers should be fair, centralized, and tiered according to type of submission specific to that technology.
10. References:
- O’Rourke B, Oortwijn W, Schuller T. The new definition of health technology assessment: A milestone in international collaboration. Vol. 36, International Journal of Technology Assessment in Health Care. Cambridge University Press; 2020. p. 187–90.
- Castro HE. Advancing HTA in Latin America: The Policy Process of Setting up an HTA Agency in Colombia. Glob Policy. 2017 Mar 1;8:97–102.
- Towse A, Devlin N, Hawe E, Garrison L. The Evolution of HTA in Emerging Markets Health Care Systems: Analysis to Support a Policy Response OHE Consulting Report for PhRMA. 2011.
- Battista RN, Hodge MJ. The natural history of health technology assessment. Vol. 25, International Journal of Technology Assessment in Health Care. 2009. p. 281–4.
- Tarricone R, Torbica A, Drummond M. Challenges in the Assessment of Medical Devices: The MedtecHTA Project. Health Economics (United Kingdom). 2017 Feb 1;26:5–12.
- Schnell-Inderst P, Mayer J, Lauterberg J, Hunger T, Arvandi M, Conrads-Frank A, et al. Health technology assessment of medical devices: What is different? An overview of three European projects. Z Evid Fortbild Qual Gesundhwes. 2015;109(4–5):309–18.
- Fuchs S, Olberg B, Panteli D, Busse R. HEALTH TECHNOLOGY ASSESSMENT of MEDICAL DEVICES in EUROPE: PROCESSES, PRACTICES, and METHODS. Int J Technol Assess Health Care. 2016;32(4):246–55.
- Ciani O, Wilcher B, Blankart CR, Hatz M, Rupel VP, Erker RS, et al. HEALTH TECHNOLOGY ASSESSMENT OF MEDICAL DEVICES: A SURVEY OF NON-EUROPEAN UNION AGENCIES. Int J Technol Assess Health Care. 2015 Jul 21;31(3):154–65.
- Fuchs S, Olberg B, Panteli D, Perleth M, Busse R. HTA of medical devices: Challenges and ideas for the future from a European perspective. Health Policy (New York). 2017 Mar 1;121(3):215–29.
- Siegfried N, Wilkinson T, Hofman K. S A H R 2 0 t h E d i t i o n S A H R 2 0 t h E d i t i o n Where from and where to for health technology assessment in South Africa? A legal and policy landscape analysis Where from and where to for health technology assessment in South Africa? A legal and policy landscape analysis [Internet]. Available from: http://www.
- O’Donnell JC, Pham S v., Pashos CL, Miller DW, Smith MD. Health technology assessment: Lessons learned from around the world – An overview. Value in Health. 2009;12(SUPPL. 2).
- Drummond MF, Schwartz JS, Jönsson B, Luce BR, Neumann PJ, Siebert U, et al. Key principles for the improved conduct of health technology assessments for resource allocation decisions. Vol. 24, International Journal of Technology Assessment in Health Care. 2008. p. 244–58.
- Lafortune L, Farand L, Mondou I, Sicotte C, Battista R. Assessing the performance of health technology assessment organizations: A framework. Int J Technol Assess Health Care. 2008 Jan;24(1):76–86.
- WHO. Main findings [Internet]. 2015. Available from: www.who.int
- Geneva. USING HEALTH TECHNOLOGY ASSESSMENT FOR UNIVERSAL HEALTH COVERAGE AND REIMBURSEMENT SYSTEMS [Internet]. 2015. Available from: www.who.int
- Wild C, Stricka M, Patera N. Guidance for the development of a National HTA-strategy. Health Policy Technol. 2017 Sep 1;6(3):339–47.
- Allen N, Pichler F, Wang T, Patel S, Salek S. Development of archetypes for non-ranking classification and comparison of European National Health Technology Assessment systems. Health Policy (New York). 2013 Dec;113(3):305–12.
- Banta D, Jonsson E. History of HTA: Introduction. Vol. 25, International Journal of Technology Assessment in Health Care. 2009. p. 1–6.
- EUnetHTA. EUnetHTA Core Model Version 3.0 for the full assessment of Diagnostic Technologies, Medical and Surgical Interventions, Pharmaceuticals and Screening Technologies [Internet]. 2016. Available from: www.htacoremodel.info/ViewHandbook.aspx
- Jacobs E, Antoine SL, Prediger B, Neugebauer E, Eikermann M. Defining the relevant outcome measures in medical device assessments: An analysis of the definition process in Health technology assessment. Int J Technol Assess Health Care. 2017;33(1):84–92.
- Drummond M, Griffin A, Tarricone R. Economic Evaluation for Devices and Drugs-Same or Different? 2009; Available from: www.ispor.org/News/articles/aug04/PEGuidelines.asp
- Tarricone R, Torbica A, Drummond M. Key Recommendations from the MedtecHTA Project. Health Economics (United Kingdom). 2017 Feb 1;26:145–52.
- Tarricone R, Torbica A, Drummond M. Challenges in the Assessment of Medical Devices: The MedtecHTA Project. Health Economics (United Kingdom). 2017 Feb 1;26:5–12.
- Daubner-Bendes R, Kovács S, Niewada M, Huic M, Drummond M, Ciani O, et al. Quo Vadis HTA for Medical Devices in Central and Eastern Europe? Recommendations to Address Methodological Challenges. Front Public Health. 2021 Jan 8;8.
- Malaysian HTA Section (MaHTAS). Health Technology Assessment Manual.
- Wild et al. 2017. Guidance for the development of an national HTA strategy.
- Lopert R, Ruiz F, Chalkidou K, Chanturidze T, Gheorghe A, Bejan A, et al. Technical Assistance for institution building of Health Technology Assessment structure, including training for the National Agency for Medicines & Medical Devices Deliverable 2: Designing an institutional framework for HTA in Romania.
